Guidelines for the Treatment of Adults with Metastatic Brain Tumors
6. Treatment Options for Adults with Multiple Metastatic Brain Tumors
Download PDF Neurosurgery, 2019
Sponsored by: The Congress of Neurological Surgeons and the Section on Tumors
Affirmation of Educational Benefit by: The Congress of Neurological Surgeons and the American Association of Neurological Surgeons
Mario Ammirati, MD, MBA,1 Brian V. Nahed, MD, MSc,2 David Andrews, MD,3 Clark C. Chen, MD, PhD,4 and Jeffrey J. Olson, MD5
- Department of Neurosurgery, St. Rita Medical Center, Lima, Ohio, USA; Department of Biology, College of Science and Technology and Sbarro Health Research Organization, Temple University, Philadelphia, Pennsylvania, USA
- Department of Neurosurgery, Massachusetts General Hospital, Boston, Massachusetts, USA
- Department of Neurosurgery, Thomas Jefferson University, Philadelphia, Pennsylvania, USA
- Department of Neurosurgery, University of Minnesota Medical School, Minneapolis, Minnesota, USA
- Department of Neurosurgery, Emory University School of Medicine, Atlanta, Georgia, USA
Correspondence:
Mario Ammirati, MD, MBA
Department of Neurosurgery
St. Rita Medical Center
770 W. High Street, Suite 220
Lima, Ohio 45801
lemoko60@me.com
Disclaimer of Liability
This clinical systematic review and evidence-based guideline was developed by a multidisciplinary physician volunteer task force and serves as an educational tool designed to provide an accurate review of the subject matter covered. These guidelines are disseminated with the understanding that the recommendations by the authors and consultants who have collaborated in their development are not meant to replace the individualized care and treatment advice from a patient's physician(s). If medical advice or assistance is required, the services of a competent physician should be sought. The proposals contained in these guidelines may not be suitable for use in all circumstances. The choice to implement any particular recommendation contained in these guidelines must be made by a managing physician in light of the situation in each particular patient and on the basis of existing resources.
Keywords:
Brain metastases, cerebral metastases, multiple metastases, radiotherapy, resection, whole brain radiation therapy
Abbreviations
COI: Conflict of interest
GKR: Gamma knife radiosurgery
MVA: Multivariate analysis
OS: Overall survival
RCT: Randomized controlled trial
RT: Radiation therapy
SRS: Stereotactic radiosurgery
WBRT: Whole brain radiation therapy
ABSTRACT
Target population: These recommendations apply to adult patients newly diagnosed with multiple (more than one) brain metastases.
Question 1: In what circumstances should whole brain radiation therapy be recommended to improve tumor control and survival in patients with multiple brain metastases?
Recommendation:
Level 2: It is recommended that whole brain radiation therapy can be added to stereotactic radiosurgery to improve local and distant control, keeping in mind the potential for worsened neurocognitive outcomes and that there is unlikely to be a significant impact on overall survival.
Question 2: In what circumstances should stereotactic radiosurgery be recommended to improve tumor control and survival in patients with multiple brain metastases?
Recommendations:
Level 1: In patients with 2 to 3 brain metastases not amenable to surgery, the addition of stereotactic radiosurgery to whole brain radiation therapy is not recommended to improve survival beyond that obtained with whole brain radiation therapy alone.
Level 3: The use of stereotactic radiosurgery alone is recommended to improve median overall survival for patients with more than 4 metastases having a cumulative volume <7 cc.
Question 3: In what circumstances should surgery be recommended to improve tumor control and survival in patients with multiple brain metastases?
Recommendation:
Level 3: In patients with multiple brain metastases, tumor resection is recommended in patients with lesions inducing symptoms from mass effect that can be reached without inducing new neurologic deficit and who have control of their cancer outside the nervous system.
INTRODUCTION
Rationale
Multiple brain metastases are found in up to 61% of patients at diagnosis.1 With the widespread use of 3T magnetic resonance imaging, it is likely that the incidence of multiple brain metastases will increase.2 In general, the presence of multiple brain metastases per se is not an indicator of an adverse prognosis compared to a single brain metastasis. Some randomized controlled trials (RCTs) show, with different degrees of robustness linked to primary endpoint selection and sample size, that overall survival is not affected by 1 versus >1 (<4) metastases.3-5 Alternatively, a prospective observational non-inferiority study showed that patients with 1 metastasis survive longer than those with 2 to 10 metastases.6 Rather, the activity of systemic disease and its propensity to be controlled represent in many studies a significant factor linked to survival.3 In many studies reporting the cause of death, systemic causes of death trump neurological causes of death.4 The goal of treatment of a patient with brain metastases, either single or multiple, is that of palliating and/or preventing neurologic symptoms, while also maintaining a good quality of life. In this context, surgery and radiation (focal or otherwise) have represented the mainstay of treatment. Lately, targeted therapies for some cancers have shown central nervous system activity, to a degree, making them a useful adjunct in the treatment of brain metastases. 7, 8
Treatment of brain metastases needs to be individualized while relying as much as possible on evidence- based guidelines. Unfortunately, Class I evidence is very rare, likely due to multiple factors, including inherent clinician bias favoring one treatment versus the other.
Objectives
With these limitations in mind, the authors undertook the task of looking at the available evidence in guiding treatment for patients with multiple brain metastases to better define the relative indications of stereotactic radiosurgery (SRS), whole brain radiation therapy (WBRT), and surgery.
METHODS
Writing Group and Question Establishment
The task force represents a multi-disciplinary panel of clinical experts encompassing neurosurgery, neurooncology, and radiation oncology. Together, participants were recruited to develop these evidence-based practice guidelines for patients with metastatic brain tumors. Questions were developed following suggestions on salient clinical questions from the collective clinical task force.
Search Method
The following electronic databases were searched for the period of January 1, 2000, to December 31, 2015: PubMed, Embase, and Cochrane CENTRAL. The search strategies used for each question can be found in Tables 1 and 2.
Study Selection and Eligibility Criteria
Eligibility Criteria
- Peer-reviewed publications,
- Patients with >1 brain metastases representing either the whole subject of the study or a subgroup of the study population, if actionable information could be extracted from it,
- Each study had >10 subjects,
- Patients >18 years of age. Studies with mixed adult and child populations were included if the adult cohorts could be isolated and analyzed separately
- Publications in English,
- Excluded radiosensitive tumor histologies (small cell lung cancer, lymphoma, and multiple myeloma).
Data Collection Process
Citations were independently reviewed and included if they met the a priori criteria for relevance. Corresponding full-text manuscripts were obtained for all citations meeting the criteria, and reviewed. Articles that did not meet the selection criteria were removed. Full-text manuscripts were more carefully reviewed to make sure there were no discrepancies in study eligibility. Data were extracted and compiled into evidence tables. The evidence tables and data were reviewed by all authors.
Evidence Classification and Recommendation Levels
The search generated a list of abstracts, which were screened. Those articles that addressed the identified questions underwent full-text independent review by the authors. Reviewers were critical in their assessment of trial design, including whether the study was retrospective, study size, randomization of treatment, baseline characteristics between study groups which could account for survivorship bias, blindness, selection bias, and appropriate statistical analyses of reported data. Studies were also evaluated as single physician experiences, single institution, or multi-institution studies. Studies were rated on the quality of the published evidence and the factors mentioned above. Level 1 recommendations were based on well-designed randomized controlled studies with clear mechanisms to limit bias. Level 2 recommendations were based on studies that were randomized control studies with design flaws, leading to bias that limited the paper’s conclusions, non-randomized cohort studies, and case-control studies. Level 3 recommendations were based on single physician, single institutional case series, comparative studies with historical control, and randomized studies with significant flaws related to under-powered studies and statistical analysis. Additional information on the method of data classification and translation to recommendation level can be found at here .
Assessment for Risk of Bias
The authors critically evaluated the studies design in terms of:
- retrospective/prospective nature,
- study size,
- randomization,
- characteristics of studies that could be related to survivorship bias or, selection bias such as single versus different primary cancer,
- appropriate statistical analysis including clear endpoint specification,
- single versus multi institutions accrual.
- Level I was reserved for well-designed randomized controlled studies with clear mechanisms to limit bias. Level II recommendations described studies that were randomized control studies with design flaws leading to bias that limits the paper’s conclusions, non-randomized cohort studies, and case-control studies. Level III recommendations were reserved for single surgeon, single institutional case series, comparative studies with historical control, and randomized studies with significant flaws related to under-powered studies and statistical analysis. Additional information on study classification and recommendation development can be found at here
RESULTS
Study Selection and Characteristics
The literature search yielded 4,228 unique articles. By reviewing the titles and/or abstracts, the authors excluded, among others, all articles referring to case reports, pediatric patients, those dealing predominantly with chemotherapy or with <10 patients, as well as articles dealing with lymphoma, small cell cancer, or myelomas. The authors were then left with 964 publications, whose abstracts/full texts were reviewed by 2 authors independently. Of these, 13 studies met the defined criteria for inclusion. Figure 1 depicts the number of studies in each part of the selection and review process.
Summary of prior recommendations
In 2009, Videtic et al9 reported on the American College of Radiology appropriateness criteria on multiple brain metastases. Videtic et al9 concluded that “WBRT is an effective palliative treatment for patients with multiple brain metastases. Approximately half of these patients experience an improvement in their neurological symptoms. However, a majority of them do not achieve local control and frequently die of progressive brain disease. Any perceived benefit from surgery needs verification in prospective, randomized, phase III clinical trials. The effectiveness of SRS for patients with multiple metastases may be primarily a function of proper patient selection but it probably cannot replace the benefits of WBRT, as demonstrated in the Aoyama trial.”
In 2012, Tsao et al10 published an updated Cochrane Review on WBRT for the treatment of newly diagnosed multiple brain metastases. The authors reported that “none of the RCTs with altered WBRT dose-fractionation schemes as compared to standard (3000 cGy in 10 daily fractions or 2000 cGy in 4 or 5 daily fractions) found a benefit in terms of overall survival, neurologic function, or symptom control. The use of radiosensitizers or chemotherapy in conjunction with WBRT remains experimental. Radiosurgery boost with WBRT may improve local disease control in selected participants as compared to WBRT alone, although survival remains unchanged for participants with multiple brain metastases. The addition of WBRT to radiosurgery improves local and distant brain control but there is no difference in overall survival. Patients treated with radiosurgery alone were found to have better neurocognitive outcomes in one trial, as compared to patients treated with WBRT and radiosurgery. The benefit of WBRT, as compared to supportive care alone, has not been studied in RCTs. It may be that supportive care alone, without WBRT, is appropriate for some participants, particularly those with advanced disease and poor performance status.”
Tsao et al11 in 2012 reported on radiotherapeutic and surgical management for newly diagnosed brain metastasis(es) in the American Society for Radiation Oncology evidence-based guideline. Tsao et al11 concluded that “multiple brain metastases and good prognosis (expected survival 3 months or more): for selected patients with multiple brain metastases (all less than 3 to 4 cm), radiosurgery alone, WBRT and radiosurgery, or WBRT alone should be considered, based on level 1 evidence. Safe resection of a brain metastasis or metastases causing significant mass effect and postoperative WBRT may also be considered (level 3).
Patients with poor prognosis (expected survival of less than 3 months): patients with either single or multiple brain metastases with poor prognosis should be considered for palliative care with or without WBRT (level 3). It should be recognized, however, that there are limitations in the ability of physicians to accurately predict patient survival. Prognostic systems such as recursive partitioning analysis, and diagnosis-specific graded prognostic assessment may be helpful.”
Sahgal et al in 201512 published a meta-analysis of phase III trials of SRS with or without WBRT for 1 to 4 brain metastases. Using individual patient data, the meta-analysis was performed on 3 prospective randomized trials comparing SRS or surgery + WBRT versus SRS/surgery alone. The authors concluded that “for patients ≤ 50 years of age, SRS alone favored survival, in addition, the initial omission of WBRT did not impact distant brain relapse rates. SRS alone may be the preferred treatment for this age group.”
In a late secondary analysis of the population from Aoyama et al 13 in 2006, it was observed that for patients with a favorable prognosis determined by high diagnosis-specific Graded Prognostic Assessment scores who had 1-4 metastases from non-small cell carcinoma of the lung the addition of WBRT to SRS resulted in a clear improvement to overall survival.
In a point-counterpoint setting published in 2015, 2 radiation oncologists gave opposing recommendations on the use of WBRT in patients with a limited number of brain metastases. Sahgal et al 14 favored withholding WBRT, while Mehta et al15 developed the opposite recommendation.
Question 1: In what circumstances should whole brain radiation therapy be recommended to improve tumor control and survival in patients with multiple brain metastases?
Class II evidence
There is 1 prospective randomized study analyzing patients with 1 to 3 brain metastases allocated to WBRT + SRS or SRS alone, designed to evaluate neurocognition (a primary endpoint).16 In this study, withholding WBRT in favor of radiosurgery alone was associated with improved neurocognition and increased survival, but decreased local and distant control. These findings need to be interpreted carefully considering that in this study WBRT was not implemented using hippocampal sparing that has been suggested in a phase II study, to reduce the neurocognitive deleterious effects of WBRT.17
Another prospective randomized study analyzed patients with 1 to 4 brain metastases treated with WBRT + SRS versus SRS alone. The primary endpoint was overall survival (OS). WBRT + SRS was no better than SRS alone in terms of OS. Local and distant failures, as well as salvage treatment were significantly less in WBRT + SRS than SRS alone. However, no difference in the cause of death between the 2 groups was detected. Multivariate analysis (MVA) showed that the presence of multiple metastases did not affect OS or the development of non-original failure in the 2 treatment groups. Overall survival was affected by age (<65 years old), primary tumor status, and extracranial disease status (MVA). Distant, non-original site metastases were affected by extracranial disease status. One observation from this publication is that the local control rate was higher in WBRT + SRS, despite the SRS dose being 30% lower in this group.4 This suggests that the dosing of SRS with and without WBRT has not been optimized yet.
Another Class II study looked at patients with 1 to 3 brain metastases treated with surgery or SRS + WBRT versus surgery or SRS alone. There was no difference in functional independence (primary endpoint). WBRT significantly decreased local failure, significantly decreased neurological death and significantly increased distant control, but had no significant effect on OS. In MVA, the number of brain metastases was not related to the primary endpoint (functional independence). Withholding WBRT does not affect functional independence.5
Although these studies are good quality, prospective, randomized studies, they are deemed to be Class II evidence, supporting Level II recommendations because they lump together single and multiple brain metastases, and because they were not designed to specifically address the value of WBRT in patients with multiple brain metastases. However, useful and actionable information may be extracted from these studies to answer the question.
Class III evidence
Multiple Class III studies, mainly observational retrospective cohort studies, suggest that radiosurgery is an effective modality to treat multiple brain metastases.18, 19 Other Class III studies suggest that WBRT is an effective tool to treat multiple brain metastases. 20, 21 As there is no preponderance of Class III evidence for SRS or WBRT alone over a broad range of circumstances no specific recommendation based on this information has been formulated.
Synthesis of Results
Class III data shows that for 2 to 4 metastases SRS can be used instead of WBRT depending on tumor volume, location, and histology and on patient functional status. For >4 metastases SRS is an option, especially when the overall volume of the lesions is clinical determined to be small. Class II data suggest that WBRT can be added in cases of multiple metastases to improve local and distant central nervous system control but may have an adverse effect on neurocognitive function and is unlikely to improve overall survival.
Question 2: In what circumstances should stereotactic radiosurgery be recommended to improve tumor control and survival in patients with multiple brain metastases?
Class I evidence
There is 1 Class I study showing that in patients with 1 to 3 brain metastases not amenable to surgery, the addition of stereotactic radiosurgery to WBRT does not improve survival compared to WBRT alone, both in the whole group or in the group with 2 to 3 brain metastases.3
Class II evidence
There is 1 prospective randomized study showing that SRS + WBRT is superior to WBRT alone in patients with 2 to 4 brain metastases in terms of local control (primary endpoint). However, this study is underpowered, and its findings relating to local control have never been replicated. 22 Although outside of the planned period of literature search, this study is included here for historical perspective from the prior sets of guidelines. Additional detail is available in the evidence tables.
Class III evidence
The results of a multi-institutional prospective observational non-inferiority study show that in patients with 2 to 4 versus 5 to 10 metastases treated with SRS, OS is the same (primary endpoint). Neurologic death, neurologic deterioration, local recurrence, and distant failure are the same in the 2 to 4 metastases group versus the 5 to 10 metastases group. The authors of the study advocate SRS, rather than WBRT, as the primary treatment for patients with <10 brain metastases.6
A single-institution retrospective cohort study identified among patients with ≥4 brain metastases treated with radiosurgery a subgroup of patients with overall combined metastatic volume of <7cc and 4 to 6 metastases with a favorable survival compared to patients with overall metastatic volume of ≥7 cc and/or ≥7 metastases.23
Two observational cohort studies, 1 retrospective and 1 prospective, have been reported in the time period examined showing that SRS is a valid treatment modality for patients with multiple brain metastases.18, 19
Synthesis of Results
A synthesis of the available data shows that it is safe and effective to use focal radiation therapy to improve local control, but not extend overall survival, in the treatment of patients with multiple brain metastases.
Question 3: In what circumstances should surgery be recommended to improve tumor control and survival in patients with multiple brain metastases?
Class III evidence
Bindal et al24 reported on a retrospective case series of 56 patients with multiple brain metastases treated with surgery and WBRT. Surgery involved resection of all metastases in 26 patients and resection of some metastases in 30 patients. The authors concluded that “…surgical removal of all lesions in selected patients with multiple brain metastases results in significantly increased survival time and gives a prognosis similar to that of patients undergoing surgery for a single metastasis.” Although outside of the planned period of literature search, and therefore not used for preparation of the recommendation, this study is included here for historical perspective from the prior sets of guidelines. Additional detail is available in the evidence tables.
Iwadate et al25 investigated, in a retrospective cohort, the role of surgery and WBRT in the treatment of 138 patients with single and multiple brain metastases. Median survival times were 8.7 months for patients with single metastases and 9.2 months for patients with multiple metastases, showing no significant difference.
Pollock et al26 reported on a retrospective case series of 52 patients with multiple brain metastases treated with a combination of WBRT, surgery, and SRS. Five patients (10%) underwent multiple simultaneous craniotomies and resection of large, symptomatic, surgically accessible metastases, while 16 patients (30%) underwent resection of only 1 metastasis. The authors concluded that “well-selected patients with multiple brain metastases appear to benefit from surgery and SRS compared to historical controls of patients treated with WBRT alone. An approach to good prognosis patients with multiple brain metastases utilizing surgical resection, SRS, and WBRT, may improve survival for this difficult patient group.”
Synthesis of Results
The use of surgery in treating multiple brain metastases may be beneficial in patients with accessible symptomatic lesions, and controlled or treatable primary disease.
DISCUSSION
Surgery may be of benefit in patients with multiple brain metastases with accessible lesions and neurological symptoms that would benefit from decompression in the context of treatable and/or controllable primary disease. Otherwise, WBRT or SRS should both be considered as valid primary therapies depending on the clinical setting and goals of therapy. They are also useful therapeutic modalities after-surgical resection.
SRS may have an advantage versus WBRT when neurocognition is assessed, although the role of the primary tumor burden on declining neurocognition may be relevant.4
Moreover, the newer WBRT delivery techniques using hippocampal avoidance may lessen the SRS advantage regarding neurocognition.17 Targeted systemic therapies are another variable to consider when individualizing therapy in patients with multiple brain metastases.7, 8
In summary, it is mandatory that the clinical team treating patients with brain metastases always be cognizant of the palliative nature of brain metastases treatment and of the paramount importance of preserving good quality life in the context of preventing, as much as possible, neurologic death.
Key Issues for Future Investigation
There is a need for robust Class I studies addressing the necessity of WBRT and the value of focal therapy (SRS and/or surgery) in patients with multiple brain metastases. Similarly, the value of targeted systemic therapy will need to be assessed, especially in patients with small and/or non-symptomatic multiple brain metastases. Regarding hippocampal avoidance WBRT, NRG CC001 is a National Cancer Institute-approved phase III trial ( https://www.nrgoncology.org/Clinical-Trials/NRG-CC001 ) that will evaluate the potential combined neuroprotective effects of hippocampal avoidance in addition to prophylactic memantine during WBRT for brain metastases.
Conflict of Interest
The Brain Metastases Guideline Update Task Force members were required to report all possible COI prior to beginning work on the guideline, using the COI disclosure form of the AANS/CNS Joint Guidelines Review Committee, including potential COIs that are unrelated to the topic of the guideline. The CNS Guidelines Committee and Guideline Task Force Chair reviewed the disclosures and either approved or disapproved the nomination. The CNS Guidelines Committee and Guideline Task Force Chair are given latitude to approve nominations of task force members with possible conflicts and address this by restricting the writing and reviewing privileges of that person to topics unrelated to the possible COIs. The conflict of interest findings are provided in detail in the companion introduction and methods manuscript (here).
Disclosures
These evidence-based clinical practice guidelines were funded exclusively by the Congress of Neurological Surgeons and the Tumor Section of the Congress of Neurological Surgeons and the American Association of Neurological Surgeons, which received no funding from outside commercial sources to support the development of this document. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
ACKNOWLEDGEMENTS
The authors acknowledge the Congress of Neurological Surgeons Guidelines Committee for its contributions throughout the development of the guideline and the American Association of Neurological Surgeons/Congress of Neurological Surgeons Joint Guidelines Review Committee for its review, comments, and suggestions throughout peer review, as well as Trish Rehring, MPH, CHES, CNS Guidelines Senior Manager, and Mary Bodach, MLIS, for her assistance with the literature searches. Throughout the review process, the reviewers and authors were blinded from one another. At this time, the guidelines task force would like to acknowledge the following individual peer reviewers for their contributions: Manish Aghi, MD, PhD, Manmeet Ahuwalia, MD, Sepideh Amin-Hanjani, MD, Edward Avila, MD, Maya Babu, MD, MBA, Kimon Bekelis, MD, Patricia Brastianos, MD, Paul Brown, MD, Andrew Carlson, MD, MS, Justin Jordan, MD, Terrence Julien, MD, Cathy Mazzola, MD, Adair Prall, MD, Shayna Rich, MD, PhD, Arjun Sahgal, MD, Erik Sulman, MD, May Tsao, MD, Michael Voglebaum, MD, Stephanie Weiss, MD, and Mateo Ziu, MD.
Figure 1. PRISMA Flow Chart
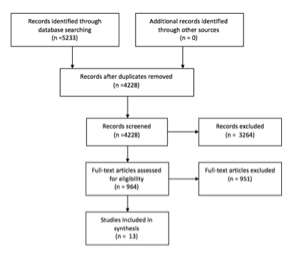
Table 1. Search Strategies for Multiple Metastases and WBRT
|
PUBMED, searched on April 19, 2016-April 20, 2016
|
|
Step 1: Brain Neoplasms [Mesh]
|
|
Step 2: (brain [TIAB] OR brainstem [TIAB] OR intracranial [TIAB]) AND (cancer [TIAB] OR tumor* [TIAB] OR tumour* [TIAB] OR neoplasm* [TIAB])
|
|
Step 3: Step #1 OR Step #2
|
|
Step 4: Neoplasm Metastasis [Mesh]
|
|
Step 5: (brain [TIAB] OR brainstem [TIAB] OR intracranial [TIAB]) AND (Metastas*) [TIAB]
|
|
Step 6: Step #4 OR Step #5
|
|
Step 7: Step #3 and Step #6
|
|
Step 8: Brain neoplasms/secondary [Mesh]
|
|
Step 9: Step #7 OR Step #8
|
|
Step 10: Cranial irradiation [Mesh]
|
|
Step 11: WBRT [TIAB]
|
|
Step 12: “whole brain” [TIAB] AND (radiotherap* [TIAB] OR radiation [TIAB] OR radiation therap* [TIAB] OR irradiation [TIAB])
|
|
Step 13: Step #10 OR Step #11 OR Step #12
|
|
Step 14: Step #9 AND Step #13
|
|
Step 15: Step #14 AND English [Lang]
|
|
Step 16: (animals [MeSH] NOT humans [MeSH]) OR case reports [PT] OR review [PT] OR comment [PT] OR letter [PT] OR editorial [PT] OR addresses [PT] OR news [PT] OR “newspaper article” [PT]
|
|
Step 17: Step #15 NOT Step #16
|
|
Step 18: Step #17 AND ("2000/01/01"[PDAT] : "2015/12/31"[PDAT])
|
|
Total: 1212 results
|
|
EMBASE, searched on April 19, 2016-April 20, 2016 :
|
|
Step 1: ‘Brain tumor’/exp
|
|
Step 2: ((brain OR brainstem OR intracranial) NEAR/3 (cancer OR tumor* OR tumour* OR neoplasm*)):ab,ti
|
|
Step 3: Step #1 OR Step #2
|
|
Step 4: ‘brain metastasis’/exp
|
|
Step 5: ((brain OR brainstem OR intracranial) NEXT/3 metastas*):ab,ti
|
|
Step 6: Step #4 OR Step #5
|
|
Step 7: Step #3 AND Step #6
|
|
Step 8: ‘brain radiation’/exp
|
|
Step 9: WBRT:ab,ti
|
|
Step 10: (‘whole brain’ NEXT/3 (radiation OR radiotherapy OR irradiation)):ab,ti
|
|
Step 11: Step #8 OR Step #9 OR Step #10
|
|
Step 12: Step #7 AND Step #11
|
|
Step 13: Step #12 AND ([article]/lim OR [conference paper]/lim) AND [humans]/lim AND [english]/lim AND [embase]/lim AND [2000-2015]/py
|
|
Step 14: #13 NOT ‘case report’/de
|
|
Total: 1060 results
|
|
COCHRANE, searched on April 19, 2016-April 20, 2016 :
|
|
Step 1: MeSH descriptor: [Brain Neoplasms] explode all trees
|
|
Step 2: ((brain OR brainstem OR intracranial) NEAR/3 (cancer OR tumor* OR tumour* OR neoplasm*)):ti,ab,kw
|
|
Step 3: Step #1 OR Step #2
|
|
Step 4: MeSH descriptor: [Neoplasm Metastasis] explode all trees
|
|
Step 5: ((brain OR brainstem OR intracranial) NEAR/3 Metastas*):ti,ab,kw
|
|
Step 6: Step #4 OR Step #5
|
|
Step 7: Step #3 AND Step #6
|
|
Step 8: MeSH descriptor: [Brain neoplasms/secondary]
|
|
Step 9: Step #7 OR Step #8
|
|
Step 10: MeSH descriptor: [Cranial irradiation] explode all trees
|
|
Step 11: WBRT:ti,ab,kw
|
|
Step 12: (‘whole brain’ NEXT/3 (radiation OR radiotherapy OR irradiation)):ti,ab,kw
|
|
Step 13: Step #10 OR Step #11 OR Step #12
|
|
Step 14: Step #9 and Step #13
|
|
Step 16: Filtered for publication year from 2000 to 2015
|
|
Total: 100 results
|
|
Summary of Primary Search
Combined from 3 database searched, de-duplicated, and non-English articles removed for total of 1,535 candidate articles
|
Table 2 . Search Strategies for Multiple Metastases and Focal Therapy
|
PUBMED, searched on May 3, 2016-May 4, 2016 :
|
|
Step 1: Brain Neoplasms [Mesh]
|
|
Step 2: (brain [TIAB] OR brainstem [TIAB] OR intracranial [TIAB]) AND (cancer [TIAB] OR tumor* [TIAB] OR tumour* [TIAB] OR neoplasm* [TIAB])
|
|
Step 3: Step #1 OR Step #2
|
|
Step 4: Neoplasm Metastasis [Mesh]
|
|
Step 5: (brain [TIAB] OR brainstem [TIAB] OR intracranial [TIAB]) AND (Metastas*) [TIAB]
|
|
Step 6: Step #4 OR Step #5
|
|
Step 7: Step #3 and Step #6
|
|
Step 8: Brain neoplasms/secondary [Mesh]
|
|
Step 9: Step #7 OR Step #8
|
|
Step 10: Radiosurgery [Mesh] OR Neurosurgical Procedures [Mesh]
|
|
Step 11: Radiosurg* [TIAB] OR radio-surg* [TIAB] OR radio surg* [TIAB] OR SRS [TIAB]
|
|
Step 12: Surg*[TIAB] OR resect*[TIAB] OR excision [TIAB] OR operati*[TIAB] OR neurosurg* [TIAB]
|
|
Step 13: Step #10 OR Step #11 OR Step #12
|
|
Step 14: Step #9 AND Step #13
|
|
Step 15: Step #14 AND English [Lang]
|
|
Step 16: (animals [MeSH] NOT humans [MeSH]) OR case reports [PT] OR review [PT] OR comment [PT] OR letter [PT] OR editorial [PT] OR addresses [PT] OR news [PT] OR “newspaper article” [PT]
|
|
Step 17: Step #15 NOT Step #16
|
|
Step 18: Step #17 AND ("2000/01/01"[PDAT] : "2015/12/31"[PDAT])
|
|
Total: 2624 results
|
|
EMBASE, searched on May 3, 2016-May 4, 2016 :
|
|
Step 1: ‘Brain tumor’/exp
|
|
Step 2: ((brain OR brainstem OR intracranial) NEAR/3 (cancer OR tumor* OR tumour* OR neoplasm*)):ab,ti
|
|
Step 3: Step #1 OR Step #2
|
|
Step 4: ‘brain metastasis’/exp
|
|
Step 5: ((brain OR brainstem OR intracranial) NEXT/3 metastas*):ab,ti
|
|
Step 6: Step #4 OR Step #5
|
|
Step 7: Step #3 AND Step #6
|
|
Step 8: ‘Radiosurgery’/exp
|
|
Step 9: ‘Stereotaxic surgery’/exp
|
|
Step 10: ‘Neurosurgery’/exp
|
|
Step 11: (Radiosurg* OR radio surg* OR SRS):ab,ti
|
|
Step 12: (Surg* OR resect* OR excision OR operati* OR neurosurg*):ab,ti
|
|
Step 13: Step #8 OR Step #9 OR Step #10 OR Step #11 OR Step #12
|
|
Step 14: Step #7 AND Step #13
|
|
Step 15: Step #14 AND ([article]/lim OR [conference paper]/lim) AND [humans]/lim AND [english]/lim AND [embase]/lim AND [2000-2015]/py
|
|
Step 16: Step #15 NOT ‘case report’/de
|
|
Total: 1060 results
|
|
COCHRANE, searched on May 3, 2016-May 4, 2016 :
|
|
Step 1: MeSH descriptor: [Brain Neoplasms] explode all trees
|
|
Step 2: ((brain OR brainstem OR intracranial) NEAR/3 (cancer OR tumor* OR tumour* OR neoplasm*)):ti,ab,kw
|
|
Step 3: Step #1 OR Step #2
|
|
Step 4: MeSH descriptor: [Neoplasm Metastasis] explode all trees
|
|
Step 5: ((brain OR brainstem OR intracranial) NEAR/3 Metastas*):ti,ab,kw
|
|
Step 6: Step #4 OR Step #5
|
|
Step 7: Step #3 AND Step #6
|
|
Step 8: MeSH descriptor: [Brain neoplasms/secondary]
|
|
Step 9: Step #7 OR Step #8
|
|
Step 10: MeSH descriptor: [Radiosurgery] explode all trees
|
|
Step 11: MeSH descriptor: [Neurosurgical Procedures] explode all trees
|
|
Step 12: (Radiosurg* OR radio-surg* OR radio surg* OR SRS):ti,ab,kw
|
|
Step 13: (Surg* OR resect* OR excision OR operati* OR neurosurg*):ti,ab,kw
|
|
Step 14: Step #10 OR Step #11 OR Step #12 or Step #13
|
|
Step 15: Step #9 AND Step #14
|
|
Step 16: Filtered for publication year from 2000 to 2015
|
|
Total: 100 results
|
|
Summary of Primary Search
Combined from 3 database searched, de-duplicated, and non-English articles removed for total of 3,698 candidate articles
|
Table 3. Evidence
|
Cho et al,19 2015
|
Retrospective cohort
Single institution817 patients with brain metastases from NSCLC treated with SRS
270 (33%) had single brain metastasis
547 (67%) > 1 metastasis
Endpoints: OS, PFS, salvage treatment-free survival
|
III
|
OS was 13 months (median): age (<65 vs ≥65), sex (male vs female), lower RPA, DS-GPA score, adenocarcinoma vs squamous cell carcinoma, synchronous vs methachronous, number × volume of tumors were associated with longer survival in MVA.
· Conclusions: “Intracranial tumor burden, reflecting the combined impact of the number of lesions and the cumulative tumor volume, is a more significant prognostic factor than tumor volume or tumor number alone. However, further studies confirming this prognostic factor should be performed. Although the cause of death was not progression of brain lesions in the majority of our patients, the brain lesions tended to be persistently progressive in most of these patients, despite repeated salvage treatment. LMS, in addition to local progression or development of new lesions, is an important pattern of failure and a neurological cause of death.”
|
|
Oehlke et al,20 2015
|
Prospective, nonrandomized cohort
Single institution
20 patients with >1 brain metastasis treated with HA WBRT
Number of brain metastases (median) 5; range 2-13
Endpoints: OS, PFS
|
III
|
OS was 71.5 weeks (median); PFS (intracranial) was 40 weeks (median)
Conclusions: “Whole brain irradiation with hippocampal sparing (SIP) and dose escalation (SIB) on multiple brain metastases is a safe and tolerable treatment regime and may provide an important improvement of tumor control compared to WBRT alone. At the same time, HA-WBRT bears the potential to minimize the treatment-related side-effect of cognitive deterioration, which cannot be reliably assessed from retrospective chart review. Accordingly, the hypothesized beneficial effect on cognition is currently under investigation in a prospective randomized phase II trial led by one of the authors.”
|
|
Yamamoto et al,6 2014
|
Prospective observational; noninferiority study; multiple institutions
Patients with 1-10 brain metastases
1 metastasis (n = 455)
2-4 metastases (n = 531)
5-10 metastases (n = 208)
Treatment: SRS
Primary endpoint: OS in patients with 5-10 versus patients with 1-4 metastases
|
II
|
· Primary endpoint: OS better for 1 metastasis but no different between 2-4 and 5-10 metastases
· Survival in patients with 5-10 metastases is not inferior to that of patients with 2-4 metastases (p < .0001)
Neurologic death (10-14% in all 3 groups), neurologic deterioration, local recurrence, distant metastases were the same in the 2-4 vs 5-10 metastases groups; LMD more in 5-10 than in 2-4 group
Conclusions:
· “To our knowledge, our study of 1194 patients is the first sufficiently powered prospective observational investigation to examine whether stereotactic radiosurgery without whole-brain radiotherapy (WBRT) as the initial treatment for patients with five to ten brain metastases is non-inferior to that for patients with two to four brain metastases in terms of overall survival. Our results show the non-inferiority of stereotactic radiosurgery without WBRT for patients with five to ten brain metastases as compared with those with two to four tumours. This result challenges the practice of inconsistent use of stereotactic radiosurgery for patients with five or more brain metastases, in whom most treatment guidelines still strongly recommended WBRT, and provides evidence in favour of offering stereotactic radiosurgery to patients with multiple brain metastases. Existing treatment guidelines for the management of patients with brain metastases might need to be revised in the near future.”
Authors’ Comments:
· Does not demonstrate that SRS is associated with longer survival than WBRT for patients with multiple metastases
· Nonrandomized study: referral bias
· Nonhomogeneous group; for example, 76% of patients had lung cancer
- Patients with 5-10 metastases had a range of volume from 0.02-3.90, so some of these tumors were very small.
· 70% of patients did not have neurologic symptoms
The usual factors affect survival in MVA, such as
· Extracranial disease status
- Neurologic symptoms
- 1 vs 2-4 metastases
|
|
Zhou et al,21 2014
|
Retrospective
Single Institution
29 NSCLC patients with 87 brain metastases treated with WBRT + SIB
no. of brain metastases (mean) 3 15 patients (52%) <3 metastases
14 patients (48%) ≥3 metastases
Endpoints: OS, PFS (intracranial)
|
III
|
· OS: 10 months (median)
· PFS (intracranial): 10 months (median)
· Male vs female, adenocarcinoma vs nonadenocarcinoma, history of EGFR-TKI treatment vs non–EGFR-TKI treatment were all associated with increased OS, both in UVA and MVA.
Conclusions:
· “WBRT plus SIB with IG-IMRT is a tolerable and effective treatment for NSCLC patients with inoperable brain metastases, especially for those with SIR score >5, number of intracranial lesions <3, and history of EGFR-TKI treatment.”
|
|
Grandhi et al,18 2012
|
Retrospective cohort
Single Institution
61 patients with ≥10 brain metastases treated with SRS
7 (11.5%) had no prior therapy
8 (13.1%) had prior SRS
22 (36.1%) had prior WBRT
16 (26.2%) had prior SRS + WBRT
8 (13.1%) had prior craniotomy
Primary endpoint: OS
|
III
|
· Primary endpoint: <14 metastases, nonmelanoma primary, controlled systemic disease, KPS ≥90, lower RPA class associated with longer survival both in UVA and MVA
· OS was 4 months (median) 6.6 months (mean) and 0.25-24 months (range)
Conclusions:
· “Our findings support a role for the use of SRS in treating select patients with extensive intracranial metastatic disease. Gamma Knife surgery, because of its minimal invasiveness and single-fraction approach, may be of particular value in this population given its limited life expectancies.”
|
|
Kocher et al,5 2011
|
Prospective randomized
Multiple institutions
Patients with 1-3 brain metastases
Treatment groups
SRS (32 with multiple mets)/surgery (2 with multiple mets) + WBRT
SRS (29 with multiple mets) /surgery (5 with multiple mets) + observation
Overall, 68 of 347 patients (19%) had multiple metastases
Prerandomization stratification for
single vs 2-3 mets
presence vs absence of extracranial disease
Surgery vs SRS
WHO PS
Primary endpoint: functional independence measured as WHO PS of ≤2
|
II
|
· Primary endpoint: no difference among different treatments
· MVA of primary endpoint: Only pretreatment WHO PS of ≤2 and absence of extracranial disease related to primary endpoint. Number of brain metastases and lung vs nonlung histology not related to primary endpoint.
· WBRT decreased local failure, increased distal control and decreased neurologic death. No effect on OS.
Conclusions:
· This study shows that after radiosurgery or surgery of a limited number of brain metastases (1-3 metastases) in patients with stable or asymptomatic solid tumor outside the brain, standard adjuvant WBRT reduces the probability of intracranial relapses from nearly 80% to approximately 50%. This effect is most pronounced after surgery, where the frequency of recurrence in the resection bed is reduced from 60% to <30%. Although it translated into a modest increase in PFS, the increased intracranial tumor control did not translate into a prolonged survival time with functional independence or into a prolonged OS time.
Authors’ Comments:
· This study demonstrates that WBRT decreases intracranial failure but this does not translate into longer independence or in longer OS.
· The data from patient with 2-3 metastases were not consistently separated from those with 1 metastasis. Additionally the small number of patients with 2 or 3 metastases limits statistical power for reaching conclusions on outcomes. For these reasons this manuscript is downgraded to class II for the purposes of this guideline.
|
|
Chang et al,16 2009
|
Prospective randomized
Single institution
Patients with 1-3 brain metastases
Treatment groups
SRS (n = 28)
40% had multiple brain metastases
SRS + WBRT (n = 30)
40% had multiple brain metastases
Primary endpoint: Neurocognition: HVLT at 4 months
|
II
|
· Interim analysis stopped trial because there was a significant probability (52 vs 24%; 96% confidence interval) SRS + WBRT patients had impairment of HVLT at 4 months vs SRS alone patients
· OS higher in SRS alone group (p = .003)
· Neurologic death not statistically different in the 2 groups while systemic death higher in SRS + WBRT ( p = .013)
· Local and distant control at 1 year higher for SRS + WBRT group (p = .01 and .02).
· Combined brain control higher for SRS + WBRT (p = .0003)
Study Conclusions:
· “...memory as assessed by HVLT–R total recall is more likely to be preserved with initial SRS alone than SRS plus WBRT… This study provides Class I evidence to support the use of SRS alone in the initial management of patients newly diagnosed with one to 3 brain metastases. Authors recommend that initial SRS alone combined with close clinical monitoring should be the preferred treatment strategy for such patients. Surgical salvage should be used for local failures, and SRS or WBRT for distant failures as indicated. This strategy is consistent with the trend towards personalized medicine and tailoring therapies, rather than applying the “one size fits all” approach of giving WBRT to all patients with brain metastasis.”
Authors’ Comments:
· Very strong study demonstrating that neurocognition is negatively affected by WBRT. However, newer ways of delivering WBRT, such as hippocampal avoidance, were not tested in the study and this is a clear limitation.
· Good data on better intracranial control when WBRT is added and good data on cause of death.
· If WBRT is better for ICC but patients live less (yet both groups are stratified for RPA, number of metastases and radioresistant histology) and die more frequently of systemic disease, maybe WBRT has negative systemic effects.
· The data from patients with 2 or 3 metastases were not consistently separated from those with 1 metastasis. As the information on disease control and neurocognition cannot be separated, this manuscript is downgraded to class II for the purposes of this guideline.
|
|
Bhatnagar et al,23 2007
|
Retrospective cohort
Single institution
205 patients (189 evaluable) with ≥4 metastases treated with GKRS as sole management (17% of patients), in combination with WBRT (46%), or after failure of WBRT (38%).
Primary endpoint: OS
|
III
|
Patients with total treatment volume <7 cc and 4-6 metastases had longer OS than patients with treatment volume <7 cc and >6 metastases or patients with treatment volume ≥7 cc (13 vs 6 months; p < .00005)
Authors’ Conclusions:
It is possible to develop a MM-RPA classification in patients with >4 metastases based on total treatment volume (and no. of metastases)
|
|
Aoyama et al,4 2006
|
Prospective randomized
Multiple institutions
Patients with 1-4 brain metastases
Treatment groups
SRS (n = 67)
34 (51%) had multiple mets
WBRT + SRS (n = 65)
34 (52%) had multiple mets
Prerandomization stratification for
single vs 2-4 mets
stable vs nonstable extracranial disease
lung vs nonlung primary
Primary endpoint: OS
|
II
|
· OS: no difference
· Cause of death: no difference
· Functional preservation: No difference
· Brain tumor recurrence (local and distant sites) less ( p < .001) in WBRT + SRS, both local and distant
· Salvage therapy less (p < .001) in WBRT + SRS
· Toxic effects of radiation Same
Conclusions:
· SRS alone without upfront WBRT was associated with increased brain tumor recurrence; however, it did not result in either worsened neurologic function or increased risk of neurologic death. With respect to patient survival, the control of systemic cancer might outweigh the frequent recurrence of brain tumors. Therefore, SRS alone could be a treatment option, provided that frequent monitoring of brain tumor status is conducted.
· The local control rate was significantly higher in the WBRT + SRS group than in the SRS alone group, despite the fact that in the WBRT + SRS group the SRS dose was 30% less. This observation lends merit to the value of fractionation, which might help overcome some radiation resistance mechanisms, such as hypoxia.
Authors’ Comments:
· Presence of multiple mets did not affect OS (MVA) or development of nonoriginal mets (MVA)
· OS was affected by age <65, primary tumor status and extracranial disease status (MVA)
· Distant, and nonoriginal site mets were affected by extracranial disease status (MVA)
· The data from patients with 2 to 4 metastases were not consistently separated from those with one metastasis. As the information on disease control could not be separated, this manuscript is downgraded to class II for the purposes of this guideline.
|
|
Andrews et al,3 2004
|
Prospective randomized
Multiple institution
Patients with 1-3 nonoperable brain metastases
Treatment groups
WBRT (n = 164)
73 with multiple metastases
WBRT + SRS (n = 167) 72 with multiple metastases
Primary endpoint: OS
|
I
|
· No difference in OS
MVA significant
· RPA1 vs RPA2 survival
· Squamous/non–small cell cancer vs others survival
UVA significant
· WBRT + SRS superior for patients with 1 metastasis survival
· WBRT + SRS have better KPS and less steroids at 6 months
Authors’ Comments:
This is a good study suggesting that “radiosurgery boost after WBRT is better than WBRT alone for surgically unresectable single brain metastasis. Because of improved performance in all patients who had radiosurgery boost. WBRT and stereotactic radiosurgery should also be considered for patients with 2 or 3 brain metastases”
|
|
Pollock et al,26 2003
|
Retrospective cohort
Single Institution
52 patients with >1 brain met treated with combination of WBRT/SRS/surgery
5 patients (10%) underwent multiple simultaneous craniotomies and resection of multiple mets
16 patients (30%) underwent single craniotomy and SRS
31 patients (60%) had SRS alone
Primary endpoint: OS
|
III
|
· Primary endpoint: RPA class 1 patients have a median survival of 19 months, class 2 of 13 and class 3 of 8 months
Authors’ Conclusions:
· “At our center, management of patients with multiple brain metastases is based primarily on three factors: extent of systemic disease, performance status, and size and number of brain tumors. Briefly, patients with progressive systemic disease or poor performance status are generally recommended to have WBRT alone unless they have symptomatic mass effect from a tumor. In those cases, patients generally undergo surgical resection followed by WBRT. Alternatively, patients with stable systemic disease and a good performance status were considered candidates for aggressive management and comprise the patients in this series. The decision as to whether a particular tumor was resected was based on tumor size and a patient’s symptoms. Patients with larger tumors and symptomatic mass effect underwent tumor resection; patients with smaller tumors not causing symptomatic mass effect had radiosurgery. Diabetic patients were given special consideration for tumor resection in order to simplify their postoperative care by minimizing the need for corticosteroids. Patients with multiple large tumors underwent simultaneous craniotomies to resect separate metastases to relieve mass effect. Patients with multiple small tumors were recommended to undergo radiosurgery”
· “Well-selected patients with multiple brain metastases appear to benefit from surgery and SRS compared to historical controls of patients treated with WBRT alone. An approach to good prognosis patients with multiple brain metastases utilizing surgical resection, SRS, and WBRT, may improve survival for this difficult patient group”
Comments:
· This is a descriptive paper. Presence of multiple brain metastases is not an absolute contraindication to surgery
· The authors describe their individualized treatment of patients with multiple brain metastases.
· This is a paper describing a treatment “philosophy”
|
|
Iwadate et al,25 2000
|
Retrospective cohort
Single Institution
77 patients with single metastasis
61 patients with >1 brain metastasis
Group A: patients with single metastasis who underwent total or subtotal resection.
Group B: patients with multiple metastases who underwent total/subtotal resection and had residual tumor < 2 cm
Group C: patients with single metastasis who underwent partial resection
Group D: all other patients with multiple metastases not falling in Group B
All patients underwent WBRT
Primary endpoint: OS
|
III
|
· Primary endpoint:
OS statistically longer for A/B groups vs C/D groups
No difference in OS between patients with single or multiple metastases
Authors conclusions
· “Surgical reduction of tumor volume which is approximately larger than 2 cm improves the efficacy of adjuvant radiation therapy and contributes to survival even in the patients with multiple brain metastases”
Authors’ Comments:
· Presence of multiple brain metastases is not an absolute contraindication to surgery
|
|
Kondziolka et al,22 1999
|
Prospective randomized
Single institution
Patients with 2-4 brain metastases
Treatment groups
WBRT (n = 14)
WBRT + GKRS (n = 13)
Primary end point: Control of brain disease
|
II
|
· Local control at 1 year 0% with WBRT alone and 92% with WBRT + GKRS (p = .0016)
· Time to failure anywhere in the brain better for WBRT + GKRS than WBRT alone (p = .002)
· Trial stopped at 60% accrual because of interim analysis results
Authors’ Comments:
· “Combined WBRT and radiosurgery for patients with two to four brain metastases significantly improves control of brain disease”
Critique
· Very few patients. Excellent (92%) local control not replicated in other studies
|
|
Bindal et al,24 1993
|
Retrospective cohort
single institution
56 patients with >1 brain met
Group A: 30 patients who had 40 lesions removed via single/multiple craniotomy at the same setting. Some lesions left unresected.
Group B: 26 patients who had 55 lesions removed via single/multiple craniotomy at the same setting. No lesion left unresected.
Group C: 26 patients with single lesion resected to serve as a control for group B
All patients underwent WBRT
Primary endpoint: OS
|
III
|
· Primary endpoint: OS statistically longer for groups B and C compared with group A. No difference in OS between Groups B and C
· MVA of Survival: only group status and systemic disease significant
Authors conclusions:
· “The authors conclude that surgical removal of all lesions in selected patients with multiple brain metastases results in significantly increased survival time and gives a prognosis similar to that of patients undergoing surgery for a single metastasis.”
· “Our guidelines for the management of patients with multiple brain metastases begin with an evaluation of the extent of systemic disease in the patient. Those patients not expected to survive for longer than 3 months due to their systemic cancer are not considered surgical candidates. Radiation therapy can palliate symptoms for this length of time and is, therefore, recommended for these patients. Patients with limited or controlled systemic cancer in whom resection of all lesions is possible are considered excellent surgical candidates. Even patients in whom all lesions cannot be removed are considered surgical candidates under certain circumstances. If one or two lesions are life-threatening or highly symptomatic, surgical removal may provide the patient an increased life span or an improved quality of life beyond that achievable by radiation therapy alone. In general, the need for multiple craniotomies should not be an important deterrent to the decision to operate.”
Authors’ Comments:
· Presence of multiple brain metastases is not an absolute contraindication to surgery
· Status of systemic disease is paramount in the decision-making progress
|
DS-GPA, diagnosis specific graded prognostic assessment; EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitor; GKRS, Gamma Knife radiosurgery; HA, hippocampal avoidance; HVLT, Hopkins Verbal Learning Test; IG-IMRT, image-guided intensity-modulated radiotherapy; KPS, Karnofsky performance status; LMD, leptomeningeal disease; LMS, leptomeningeal spread; MM, multiple metastases; MVA, multivariate analysis; NSCLC, non–small cell lung cancer; OS, overall survival; PFS, progression-free survival; PS, performance status; RPA, recursive partitioning analysis; RPA 1, recursive partitioning analysis class 1; RPA 2, recursive partitioning analysis class 2; SIB, simultaneous integrated boost; SIP, simultaneous integrated protection; SIR, score index for radiosurgery in brain metastases; SRS, stereotactic radiosurgery; UVA, univariate analysis; WBRT, whole brain radiation therapy; WHO, World Health Organization.
REFERENCES
1. Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Current oncology reports. Feb 2012;14(1):48-54.
2. Tabouret E, Chinot O, Metellus P, Tallet A, Viens P, Goncalves A. Recent trends in epidemiology of brain metastases: an overview. Anticancer research. Nov 2012;32(11):4655-4662.
3. Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet (London, England). May 22 2004;363(9422):1665-1672.
4. Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. Jama. Jun 7 2006;295(21):2483-2491.
5. Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. Jan 10 2011;29(2):134-141.
6. Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. The Lancet. Oncology. Apr 2014;15(4):387-395.
7. Renfrow JJ, Lesser GJ. Molecular subtyping of brain metastases and implications for therapy. Current treatment options in oncology. Dec 2013;14(4):514-527.
8. Lin NU. Targeted therapies in brain metastases. Current treatment options in neurology. Jan 2014;16(1):276.
9. Videtic GM, Gaspar LE, Aref AM, et al. American College of Radiology appropriateness criteria on multiple brain metastases. International journal of radiation oncology, biology, physics. Nov 15 2009;75(4):961-965.
10. Tsao MN, Lloyd N, Wong RK, et al. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. The Cochrane database of systematic reviews. Apr 18 2012(4):Cd003869.
11. Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Practical radiation oncology. 2012;2(3):210-225.
12. Sahgal A, Aoyama H, Kocher M, et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: Individual patient data meta-analysis. International Journal of Radiation Oncology Biology Physics. 2015;91(4):710-717.
13. Aoyama H, Tago M, Shirato H. Stereotactic Radiosurgery With or Without Whole-Brain Radiotherapy for Brain Metastases: Secondary Analysis of the JROSG 99-1 Randomized Clinical Trial. JAMA oncology. Jul 2015;1(4):457-464.
14. Sahgal A. Point/Counterpoint: Stereotactic radiosurgery without whole-brain radiation for patients with a limited number of brain metastases: the current standard of care? Neuro-oncology. Jul 2015;17(7):916-918.
15. Mehta MP. The controversy surrounding the use of whole-brain radiotherapy in brain metastases patients. Neuro Oncol. Jul 2015;17(7):919-923.
16. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. The Lancet. Oncology. Nov 2009;10(11):1037-1044.
17. Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. Dec 1 2014;32(34):3810-3816.
18. Grandhi R, Kondziolka D, Panczykowski D, et al. Stereotactic radiosurgery using the Leksell Gamma Knife Perfexion unit in the management of patients with 10 or more brain metastases. Journal of neurosurgery. Aug 2012;117(2):237-245.
19. Cho KR, Lee MH, Kong DS, et al. Outcome of gamma knife radiosurgery for metastatic brain tumors derived from non-small cell lung cancer. Journal of neuro-oncology. Nov 2015;125(2):331-338.
20. Oehlke O, Wucherpfennig D, Fels F, et al. Whole brain irradiation with hippocampal sparing and dose escalation on multiple brain metastases: Local tumour control and survival. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft ... [et al]. Jun 2015;191(6):461-469.
21. Zhou L, Liu J, Xue J, et al. Whole brain radiotherapy plus simultaneous in-field boost with image guided intensity-modulated radiotherapy for brain metastases of non-small cell lung cancer. Radiation oncology (London, England). 2014;9:117.
22. Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. International journal of radiation oncology, biology, physics. Sep 01 1999;45(2):427-434.
23. Bhatnagar AK, Kondziolka D, Lunsford LD, Flickinger JC. Recursive partitioning analysis of prognostic factors for patients with four or more intracranial metastases treated with radiosurgery. Technology in cancer research & treatment. Jun 2007;6(3):153-160.
24. Bindal RK, Sawaya R, Leavens ME, Lee JJ. Surgical treatment of multiple brain metastases. Journal of neurosurgery. Aug 1993;79(2):210-216.
25. Iwadate Y, Namba H, Yamaura A. Significance of surgical resection for the treatment of multiple brain metastases. Anticancer research. Jan-Feb 2000;20(1b):573-577.
26. Pollock BE, Brown PD, Foote RL, Stafford SL, Schomberg PJ. Properly selected patients with multiple brain metastases may benefit from aggressive treatment of their intracranial disease. Journal of neuro-oncology. 2003;61(1):73-80.
Source: Neurosurgery