Guidelines for the Treatment of Adults with Metastatic Brain Tumors
2. The Role of Surgical Resection
Download PDF Neurosurgery, 2019
Sponsored by: The Congress of Neurological Surgeons and the Section on Tumors
Affirmation of Educational Benefit by: The Congress of Neurological Surgeons and the American Association of Neurological Surgeons
Brian V. Nahed, MD, MSc,1* Christopher Alvarez-Breckenridge, MD, PhD,1* Priscilla K. Brastianos, MD,2 Helen Shih, MD, MS, MPH,3 Andrew Sloan, MD,4 Mario Ammirati MD, MBA,5 John S. Kuo, MD, PhD,6 Timothy C. Ryken, MD,7 Steven N. Kalkanis, MD,8 and Jeffrey J. Olson, MD9
- Department of Neurosurgery, Massachusetts General Hospital, Boston, Massachusetts, USA
- Department of Neurology, Massachusetts General Hospital, Boston, Massachusetts, USA
- Department of Radiation Oncology, Massachusetts General Hospital, Boston, Massachusetts, USA
- Department of Neurosurgery, Case Western Reserve University, Cleveland, Ohio, USA
- Department of Neurosurgery, St. Rita Medical Center, Lima, Ohio, USA
- Department of Neurosurgery and Mulva Clinic for the Neurosciences, Dell Medical School, University of Texas at Austin, Austin, Texas, USA
- Section of Neurosurgery, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire, USA
- Department of Neurosurgery, Henry Ford Health System, Detroit, Michigan, USA
- Department of Neurosurgery, Emory University School of Medicine, Atlanta, Georgia, USA
Correspondence:
Brian V. Nahed, MD, MSc
Massachusetts General Hospital
Department of Neurosurgery
15 Parkman Street
Wang 745
Boston, Massachusetts 02114
bnahed@partners.org
Disclaimer of Liability
This clinical systematic review and evidence-based guideline was developed by a multidisciplinary physician volunteer task force and serves as an educational tool designed to provide an accurate review of the subject matter covered. These guidelines are disseminated with the understanding that the recommendations by the authors and consultants who have collaborated in their development are not meant to replace the individualized care and treatment advice from a patient's physician(s). If medical advice or assistance is required, the services of a competent physician should be sought. The proposals contained in these guidelines may not be suitable for use in all circumstances. The choice to implement any particular recommendation contained in these guidelines must be made by a managing physician in light of the situation in each particular patient and on the basis of existing resources.
Keywords:
Brain metastases, cerebral metastases, chemotherapy, intracranial metastatic disease, observation, radiation, recurrent metastatic brain tumors, surgery
Abbreviations
ECOG: Eastern Cooperative Oncology Group
GTR: Gross total resection
KPS: Karnofsky performance status
LMD: Leptomeningeal disease
MTR: Microscopic total resection
RPA: Recursive partitioning analysis
SRS: Stereotactic radiosurgery
STR: Subtotal resection
WBRT: Whole brain radiation therapy
No part of this manuscript has been published or submitted for publication elsewhere.
ABSTRACT
Target population: These recommendations apply to adult patients with newly diagnosed metastatic brain tumors, excluding radiosensitive tumor histologies.
Surgery for metastatic brain tumors at new diagnosis
Question: Should patients with newly diagnosed metastatic brain tumors undergo surgery, stereotactic radiosurgery (SRS), or whole brain radiation therapy (WBRT)?
Recommendations:
Level 1: Surgery + WBRT is recommended as first-line treatment in patients with single brain metastases with favorable performance status and limited extracranial disease to extend overall survival, median survival, and local control.
Level 3: Surgery + SRS is recommended to provide survival benefit in patients with metastatic brain tumors
Level 3: Multimodal treatments including either surgery + WBRT + SRS boost or surgery + WBRT are recommended as alternatives to WBRT + SRS in terms of providing overall survival and local control benefits.
Surgery and radiation for metastatic brain tumors
Question: Should patients with newly diagnosed metastatic brain tumors undergo surgical resection followed by WBRT, SRS, or another combination of these modalities?
Recommendations:
Level 1: Surgery + WBRT is recommended as superior treatment to WBRT alone in patients with single brain metastases.
Level 3: Surgery + SRS is recommended as an alternative to treatment with SRS alone to benefit overall survival.
Level 3: It is recommended that SRS alone be considered equivalent to surgery + WBRT.
Target population: These recommendations apply to adult patients diagnosed with recurrent, non-radiosensitive metastatic brain tumors.
Surgery for recurrent metastatic brain tumors
Question: Should patients with recurrent metastatic brain tumors undergo surgical resection?
Recommendation:
Level 3: Craniotomy is recommended as a treatment for intracranial recurrence after initial surgery or SRS.
Surgical technique and recurrence
Question A: Does the surgical technique (en bloc resection or piecemeal resection) affect recurrence?
Recommendation:
Level 3: En bloc tumor resection, as opposed to piecemeal resection, is recommended to decrease the risk of postoperative leptomeningeal disease when resecting single brain metastases.
Question B: Does the extent of surgical resection (gross total resection or subtotal resection) affect recurrence?
Recommendation:
Level 3: Gross total resection is recommended over subtotal resection in recursive partitioning analysis Class I patients to improve overall survival and prolong time to recurrence.
INTRODUCTION
Rationale
Surgery is recommended for brain metastases that are large, have significant perilesional edema, result in neurological deficits, and present with uncertain pathology. In addition, surgery provides tissue diagnosis, when needed. Smaller targeted craniotomies and an emphasis on minimizing postoperative deficits have led to faster operations and discharge a few days after a craniotomy. Given the limitations of radiation therapy and other targeted therapies, surgery plays a critical role for patients, the timing of which is discussed in this guideline.
METHODS
Writing Group and Question Establishment
The writers represent a multi-disciplinary panel of clinical experts encompassing neurosurgery, neuro-oncology, and radiation oncology. Together, they were recruited to develop these evidence-based practice guidelines for surgery for metastatic brain tumors. Questions were developed following salient clinical questions from the collective clinical panel. Questions were framed to build upon prior surgical guidelines for brain metastases and incorporate new developments in the field.
Literature Review
The following electronic databases were searched from January 1, 2008 to December 31, 2015: PubMed and Ovid Medline, using relevant MeSH and non-MeSH terms, including: “Metastasis”, “Metastases”, “Metastatic”, “Metastasize”, “Surgery”, “Surgical”, “Operative”, “Resect”, “Brain”, and “Brain Neoplasm.” See Appendix A for the complete search strategies.
Article Inclusion and Exclusion Criteria
Eligibility Criteria
- Peer-reviewed publications.
- Patients with newly diagnosed and recurrent brain metastases who have had surgery.
- Each study had >5 or more subjects.
- Patients
- Publications in English.
- Excluded radiosensitive tumor histologies (small cell lung cancer, lymphoma, and multiple myeloma).
Study selection and quality assessment
The search criteria were developed and abstract review was performed by two independent reviewers. Citations were independently reviewed and included if they met the a priori criteria for relevance. No discrepancies in study eligibility were noted. Corresponding full-text PDFs were obtained for all citations meeting the criteria, and were reviewed. Data were extracted by the first reviewer and verified by another, all of which were compiled into evidence tables. The tables and data were reviewed by all of the authors. Articles that did not meet the selection criteria were removed.
Evidence Classification and Recommendation Levels
Each reviewer independently determined the strength of the evidence, classified it according to the criteria described above, and a consensus level of recommendation was achieved. Additional information on the method of data classification and translation to recommendation level can be found here.
Guideline Development Process
Assessment for Risk of Bias
The literature search generated a list of abstracts, which were screened, and those articles that addressed the identified questions underwent full manuscript independent review by the authors. Reviewers were critical in their assessment of trial design, including whether the study was retrospective, a single surgeon cohort, study size, randomization of treatment, baseline characteristics between study groups that could account for survivorship bias, blindness, selection bias, and appropriate statistical analyses of reported data. Studies were also evaluated as single surgeon experiences, single institution, or multi-institution studies. Given the diversity in primary sites of metastatic brain tumors, articles were screened for their conclusions as they related to a single type of brain metastasis (eg, melanoma) or brain metastases in general (eg, lung, breast, and melanoma combined into one group). Studies were rated on the quality of the published evidence and the factors mentioned above. Level I was reserved for well-designed randomized controlled studies with clear mechanisms to limit bias. Level II recommendations described studies that were randomized control studies with design flaws leading to bias that limits the paper’s conclusions, non-randomized cohort studies, and case-control studies. Level III recommendations were reserved for single surgeon, single institutional case series, comparative studies with historical control, and randomized studies with significant flaws related to under-powered studies and statistical analysis. Additional information on study classification and recommendation development can be found here.
RESULTS
Study Selection and Characteristics
The search criteria yielded 1060 publications, which were reviewed by two authors independently. Of these, 121 studies met the eligibility criteria and were screened for inclusion. Of these, 32 studies met the criteria and specifically focused on surgery for metastatic brain tumors either at initial diagnosis or at recurrence. Figure 1 depicts the number of studies in each part of the selection and review process.
Summary of Prior Recommendations
In the previously published guidelines on surgery for the management of newly diagnosed brain metastases, two questions were answered by Level 1 recommendations. First, the question of surgical resection plus WBRT versus surgical resection alone, Kalkanis et al.1 concluded that surgery followed by WBRT represented a superior treatment modality in terms of improving tumor control at the original site of metastasis and in the brain when compared to surgical resection alone. Second, for the question of surgical resection plus WBRT versus WBRT alone, Kalkanis et al.1 concluded surgery plus WBRT is superior in patients with good performance status and limited extracranial disease.
Should patients with newly diagnosed metastatic brain tumors undergo surgery, stereotactic radiosurgery, or whole brain radiation therapy?
Results of Individual Studies, Discussion of Study Limitations, and Risk of Bias
Multiple Class III retrospective studies investigated the question of surgery versus radiation therapy as a first-line treatment for newly diagnosed brain metastases. Among these studies across various metastatic histologies, surgery resulted in significant2-10 or nearly significant11, 12 improvement in overall survival compared to either whole brain radiation therpay (WBRT) or stereotactic radiosurgery (SRS). These results were distributed among studies investigating single 3, 4, 9 and multiple brain metastases.5-8, 10-12 In these studies, patients were treated with either surgery alone8, 9, 12 or surgery plus radiation therapy. Combinations of surgery and radiation therapy included WBRT,3, 4, 6, 11 SRS,7, 12 or a combination of approaches.2, 5, 10, 13 Lindvall et al4 compared surgery plus WBRT to hypofractionated stereotactic irradiation. Surgery plus WBRT for small tumors (volumes <10 cc) may provide a survival advantage, particularly in areas of non-eloquent brain.
Several retrospective Class III studies have identified factors to consider prior to proceeding with surgery. Low Karnofsky Performance Status (KPS) was associated with poor surgical outcome in multiple studies.3, 14-16 Two Class III studies demonstrated that surgery as part of a multimodal treatment was non-inferior to WBRT plus SRS. Rades et al13 performed a matched pair analysis of 92 patients across various histologic subtypes to demonstrate equivalent 1-year local control, 1-year intracerebral control, and 1-year survival between surgery plus WBRT plus radiation boost and WBRT plus SRS. Additionally, the retrospective analysis by d’Agostino et al17 evaluated surgery plus WBRT compared to WBRT plus SRS and yielded similar rates of local control or overall survival at 1 or 5 years, suggesting equivalence of both approaches. However, the authors failed to account for tumor size or control of extracranial disease between groups, making the interpretation of these results challenging. Examples of additional limitations from these studies include treatment group imbalances,2, 6, 12 retrospective analyses,2-5, 7 non-randomization into surgical versus radiation treatment groups, variations in adjuvant therapies,9 small study size,2, 7, 8 combination of multiple tumor histologies into a single brain metastases group,3, 4 lack of control for tumor location,2, 3 lack of consideration of tumor size in enrollment criteria,3 and incomplete statistical analyses.5
Synthesis of Results
Consistent with previously published guidelines by Kalkanis et al.,1 surgery plus WBRT has been re-demonstrated as a superior treatment modality to WBRT alone.2, 3, 6. Surgery plus SRS was superior to SRS alone in multiple studies. The data for surgery versus SRS alone were conflicting8, 9, 12 and was explained in part by treatment selection bias inherent in retrospective analyses. Similar uncertainty was seen in the comparison between surgery plus WBRT and SRS alone.11. Additionally, Baykara et al6demonstrated improved overall survival in the surgery plus WBRT group compared with WBRT plus SRS, although additional studies are warranted to validate the superiority of either treatment approach. Also the strength of the conclusions about the value of combinations of these modalities is limited by the lack of randomized controlled trials addressing these questions.
Should patients with newly diagnosed metastatic brain tumors undergo surgical resection followed by WBRT, SRS, or other combination of these modalities?
Results of Individual Studies, Discussion of Study Limitations, and Risk of Bias
Two Class III studies indicate that surgery followed by WBRT results in improvement in median survival6, 18 and local failure relapse-free survival6 for surgery combined with WBRT compared to WBRT alone. However, both studies were limited in their imbalance between treatment groups 6 or lack of baseline characteristics between treatment groups.18 There are 2 Class II and 5 Class III studies to support a benefit for surgery followed by WBRT,6, 11, 17-19 SRS,20, 21 or WBRT plus SRS.20, 21 In contrast, the data for surgery followed by WBRT compared to SRS alone are less clear. The studies of Muacevic et al19 and Marko et al11 failed to demonstrate a difference between these 2 groups in terms of overall survival. However, the study by Marko et al11 demonstrated a trend towards improved mean survival in patients treated with surgery plus WBRT compared with SRS alone (20.1 months vs 12.3 months, p = .07). Surgery combined with WBRT compared with WBRT plus SRS was equivalent between groups.17 The retrospective study by d’Agostino et al17 failed to demonstrate a difference in local control or overall survival at 1 or 5 years but also failed to demonstrate an association between traditional prognostic factors and overall survival.
In a matched pair analysis for patients with 1 to 2 brain metastases, patients undergoing surgery with WBRT and an SRS boost had similar median survival, 1-year survival, and 1-year local control compared to patients undergoing WBRT and SRS.21 Similarly, Wang et al20 demonstrated in a retrospective analysis of 528 patients that surgery combined with SRS and WBRT resulted in improved overall survival compared to SRS alone on multivariate analysis but was equivalent to SRS plus WBRT or surgery plus SRS.
Synthesis of Results
Consistent with previously published guidelines by Kalkanis et al.,1 surgery plus WBRT has been re-demonstrated as a superior treatment modality to WBRT alone.2, 3, 6 Although Class III published reports suggest the benefit of surgery plus WBRT compared with WBRT alone,6, 18 findings of surgery plus WBRT compared to multimodal radiation approaches was conflicting and underpowered in class II and III studies.6, 13, 17, 19 Similarly, surgery plus SRS was shown to be superior to SRS alone7, 10, 20 but superiority among surgery plus SRS, SRS plus WBRT, or surgery plus SRS plus WBRT was not demonstrated. These findings suggest a lack of overarching evidence to support surgery plus SRS or surgery plus WBRT compared to multi-modal radiation approaches and requires interpretation of clinical features such as performance status, number of brain metastases, intracranial tumor location, and control of extracranial disease.
Should patients with recurrent metastatic brain tumors undergo surgical resection?
Results of Individual Studies, Discussion of Study Limitations, and Risk of Bias
Two Class III studies found a benefit for the role of reoperation for recurrence after an initial craniotomy for metastatic disease.22, 23 Three Class III studies have suggested a role for surgery following failed stereotactic radiotherapy.24-26 Although a time interval between SRS and resection of ≥3 months was associated with improved overall survival,24 these findings raise the concern that these patients with delayed recurrence are biased to have improved overall survival compared to short-term SRS failure. Additionally, patients with viable tumor on resection had a decreased mean survival in contrast to those patients with radiation necrosis,25 suggesting that surgery can be useful in distinguishing tumor recurrence from pseudo-progression and its associated impact on overall survival, but did not provide a comparison between surgery for recurrence compared to other treatment modalities.
Synthesis of Results
Although craniotomy for recurrence was associated with improved survival, attention should be given to preoperative functional status, age, extracranial disease, and the interval between SRS and resection.22, 24. In particular, the role of surgery for recurrence in patients >65 years of age or with an interval between SRS and surgery of <3 months is uncertain. Additionally, Obermueller et al26 suggest that surgery for recurrence after radiation in either eloquent or non-eloquent cortex leads to a higher risk of postoperative deficits. These results suggest that additional studies are warranted to investigate how resection following radiation therapy affects patients in terms of quality of life and distinguishes radiation necrosis from tumor recurrence by providing diagnostic information to guide future therapy. Moreover, these findings demonstrate the need to systemically investigate novel treatments, such as laser interstitial thermal therapy for recurrent disease that is refractory to SRS and that is located in surgically inaccessible areas.
Does surgical technique (en bloc resection or piecemeal resection) affect recurrence? Does the extent of surgical resection (gross total resection or subtotal resection) affect recurrence?
Results of Individual Studies, Discussion of Study Limitations, and Risk of Bias
En bloc resection or piecemeal resection
Three Class III studies demonstrate en bloc resection to be superior to piecemeal resection and a decreased risk of leptomeningeal disease (LMD) in single melanoma brain metastases located in the lateral ventricle,27 improved overall survival,28 a lower complication rate,29 and local recurrence, particularly in tumors < 9.71cm3.30 However, Patel et al30 demonstrated that the median volume of tumors resected by a piecemeal approach was 15.87 cm3 compared with 7.59 cm3 for en bloc resection, suggesting that these non-standardized treatment groups and associated technical limitations may have biased these results. Additional limitations from Patel et al29 were reflected in the retrospective design. For instance, there were significant differences between treatment groups requiring statistical correction, and the authors were unable to assess 30-day postoperative KPS due to incomplete clinical documentation, and there were limitations in accounting for surgical limitations that could prevent en bloc resection in eloquent cortex.
Gross total resection or subtotal resection
Consistent with the advantages of en bloc resection, gross total resection (GTR) was shown to be generally superior to subtotal resection (STR) in terms of median survival7, 26, 31 and time to local recurrence.7, 32 Of note, the improved overall survival demonstrated by Lee et al31 was found in recursive partitioning analysis (RPA) Class I patients with KPS ³70 and age <65 years with controlled primary and no extracranial metastases. There was a significant improvement in median survival for GTR plus SRS (14.1 months) compared with either STR plus SRS (7.1 months) or SRS alone (6.9 months) (p = .032).7 LMD was not associated with en bloc nor subtotal resection on univariate analysis.12 A potential limitation of studies looking at GTR and en bloc resection is the role of infiltrating tumor cells beyond the border of a brain metastasis. To address this, a Class III study found that microscopic total resection (MTR) was associated with improved local control and decreased local recurrence, but was not associated with improved overall survival compared to GTR.33
Synthesis of Results
Several studies have directly examined the role of en bloc resection and GTR in terms of improved overall survival, fewer postoperative complications, reduction of LMD, and time to local recurrence. The literature supports resection of brain metastases with the goal of GTR ideally through an en bloc approach. Future studies are warranted to investigate the role of surgical approach and LMD. In particular, identification of surgical patients who are at highest risk of developing LMD is needed. This may include tumor location, histology, and tumor features (solid, cystic, or encapsulated) and the development of techniques to reduce the risk of LMD in high-risk groups. Clinical judgment is critical to application of these considerations when the tumor resides in eloquent cortex. Additionally, prospective studies are needed to evaluate the benefit of GTR through en bloc resection for multiple brain metastases, to differentiate across multiple RPA classes, and to investigate MTR to target infiltrating tumor cells.
SUMMARY AND DISCUSSION
Multiple retrospective studies demonstrated the benefit of initial surgery compared with radiation therapy alone, particularly in patients with KPS > 70,2 younger age,7 favorable RPA class,5 and lower Eastern Cooperative Oncology Group (ECOG) score,7 control of primary tumor,8 brain metastases diameter < 4 cm,9 and complete surgical resection.7 However, conclusions regarding these findings were limited due to the lack of high-quality randomized controlled trials.
The findings of Rades13 (Class II) and D’Agostino17 (Class III) raise further questions about the role of surgery followed by adjuvant SRS and WBRT compared to WBRT plus SRS. Although a multimodal surgical approach was non-inferior to WBRT plus SRS, further studies are warranted to understand the appropriate use of surgery in terms of the number of brain metastases, tumor location, and optimal timing between surgery and adjuvant radiotherapies. Lastly, Lindvall et al raised a point regarding optimal tumor size for radiation therapy versus surgery. Although smaller tumors are typically targeted with radiotherapy rather than surgery, these authors demonstrated that surgery plus WBRT was superior to hypofractionated stereotactic irradiation for tumors <10 cc. These findings suggest that surgery plus WBRT should be considered for smaller lesions in non-eloquent cortex. The validity of these findings in a randomized controlled study is warranted, particularly given the risk of neurotoxicities associated with WBRT and the increasing use of SRS among neuro-oncologists and radiation oncologists. In particular, attention should be given towards surgery alone compared with surgery plus adjuvant SRS or surgery plus multimodal SRS + WBRT radiotherapy, as well as a determination of a lower tumor volume threshold for surgical resection.
The role of surgery for recurrence warrants further investigation with delineation between surgery and SRS as the initial treatment modality. In particular, there is a propensity towards treating patients with SRS in the setting of tumor in eloquent cortex, smaller tumor size, and an increased number of brain metastases. A current NRG study is attempting to control for these factors in a randomized fashion in order to determine if the role of surgery is most beneficial after initial surgical resection22, 23 rather than initial SRS.26 As future developments in radiographic imaging help clarify pseudo-progression following SRS, it will guide in surgical decision making with respect to concern for tumor recurrence.
Surgical technique, particularly piecemeal versus en bloc resection and GTR versus STR, was addressed in several studies. Collectively, these analyses found that en bloc resection and GTR were superior surgical approaches and that piecemeal resection was associated with an increased risk of LMD. A limitation of these studies, however, was the difference in initial tumor size between piecemeal and en bloc resection. Given limitations based on tumor size and location, an en bloc resection may not be feasible and may predispose a patient to an increased risk of postoperative complications. In addition to controlling for these factors, future studies are needed to study the role of adjuvant radiation therapy (SRS, WBRT, or both) in the setting of en bloc and piecemeal resection.
CONCLUSIONS AND KEY ISSUES FOR FUTURE INVESTIGATION
Looking towards the future, the authors found that there were several topics that were not adequately addressed in the literature. In particular, studies typically included patients with 1 to 4 brain metastases who had surgery for the largest or symptomatic lesion. Although initial publications are encouraging, additional studies are necessary to establish the settings in which there is value in the routine use of surgical resection of two or more metastases. Several studies investigated the role of surgery for recurrence after SRS or initial surgery. However, there is a lack of studies examining the role of synchronous surgical resection for multiple intracranial metastases, as well as a lack of studies examining the appropriate adjuvant radiation regimen for patients undergoing resection of these lesions.
An additional area of interest is the role of surgery in patients undergoing immunotherapy for brain metastases. Lonser et al. presented an initial retrospective analysis of patients with metastatic melanoma treated with surgery and immunotherapy (interleukin-2 [IL-2], IL-12, immunotoxin, vaccine, adoptive cell therapy, and monoclonal antibody).34 Among the cohort, adjuvant WBRT in 36% of the patients was not associated with improved survival, local, or distant brain recurrence rates. However, these findings warrant further attention as novel immunotherapeutic approaches are being applied to brain metastases. Additionally, the role of SRS, WBRT, and the combination of both adjuvant agents have not been investigated in the setting CTLA-4 and PD-1 blockade.
Advances in the management of metastatic brain tumors have led to better outcomes and longer survival. Surgery plays a large role at initial diagnosis and recurrence. Future investigation into the timing of when and how often to perform surgery while taking into account newer chemotherapeutic/immunological regimens, and radiation therapy, especially at recurrence, is critical to clearly define the role of surgery with respect to progression-free and overall survival. Lastly, emerging surgical techniques including laser interstitial therapy and minimally invasive tubular approaches are emerging surgical techniques that warrant investigation for single versus multiple brain metastases, time to adjuvant therapy, need for post-operative immunosuppressants, optimal tumor locations, and quality of life metrics as compared with conventional craniotomy.
Conflict of Interest (COI)
The Update Brain Metastases Guidelines Task Force members were required to report all possible COIs prior to beginning work on the guideline, using the COI disclosure form of the AANS/CNS Joint Guidelines Review Committee, including potential COIs that are unrelated to the topic of the guideline. The CNS Guidelines Committee and Guideline Task Force Chair reviewed the disclosures and either approved or disapproved the nomination. The CNS Guidelines Committee and Guideline Task Force Chair are given latitude to approve nominations of Task Force Members with possible conflicts and address this by restricting the writing and reviewing privileges of that person to topics unrelated to the possible COIs. The conflict of interest findings are provided in detail in the companion introduction and methods manuscript.
Disclosures
These evidence-based clinical practice guidelines were funded exclusively by the Congress of Neurological Surgeons and the Tumor Section of the Congress of Neurological Surgeons and the American Association of Neurological Surgeons, which received no funding from outside commercial sources to support the development of this document.
ACKNOWLEDGEMENTS
The authors acknowledge the Congress of Neurological Surgeons Guidelines Committee for its contributions throughout the development of the guideline and the American Association of Neurological Surgeons/Congress of Neurological Surgeons Joint Guidelines Review Committee for its review, comments, and suggestions throughout peer review. The authors would also like to acknowledge the significant contributions of Mary Bodach and Trish Rehring, as well as Martha Stone and Lisa Philpotts, medical research librarians. Throughout the review process, the reviewers and authors were blinded from one another. At this time, the guidelines task force would like to acknowledge the following individual peer reviewers for their contributions: Manish Aghi, MD, PhD, Manmeet Ahuwalia, MD, Sepideh Amin-Hanjani, MD, Edward Avila, MD, Maya Babu, MD, MBA, Kimon Bekelis, MD, Paul Brown, MD, Andrew Carlson, MD, MS, Justin Jordan, MD, Terrence Julien, MD, Cathy Mazzola, MD, Adair Prall, MD, Shayna Rich, MD, PhD, Arjun Sahgal, MD, Erik Sulman, MD, May Tsao, MD, Michael Voglebaum, MD, Stephanie Weiss, MD, and Mateo Ziu, MD.
Figure 1: PRISMA flowchart
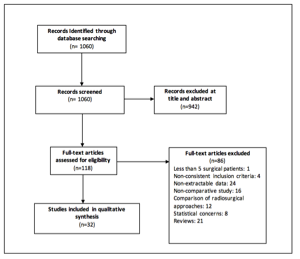
Table 1: Evidence table
| Author, Year |
Study Description |
Data Class |
Conclusion |
| Bougie et al,9 2015 |
Retrospective single institution study of 115 patients with a single brain metastasis from non–small cell lung cancer who were treated initially with either surgery (43 patients) or SRS (72 patients) |
III |
The SRS cohort on average had smaller tumors (4.4 mL) compared with the surgery cohort (25.3 mL). Local control was the same between groups. Median survival for surgical group was 13.3 months compared with 7.8 months for SRS (p = .047). In multivariate analysis of the surgical group, brain metastasis diameter 20 Gy to the margin was associated with improved local control (p = .007). Of note, patients in both groups received variable adjuvant therapies for local and distant recurrences. |
| Patel et al,29 2015 |
Single institution retrospective analysis of 1033 patients undergoing resection of a previously untreated single brain metastasis. Patients underwent either en bloc resection (62%) or piecemeal resection (38%) |
III |
There were significant differences between the two groups, including preoperative tumor volume, KPS, tumor functional grade, preoperative tumor volume, hemorrhagic tumor, cystic tumor, and symptoms. The 1-month mortality between groups was similar between groups. The complication rate for en bloc resection was 13%, compared to 19% for piecemeal resection (p = .007), and for major complication rates were 7% vs 10% between the two groups (p = .04). These differences were significant on multivariate analysis. The 30-day neurologic complication rate for piecemeal resection was 13% compared to 8% for en bloc resection (p = .03); however, the incidence of major neurologic complications was similar between groups. The incidence of overall complications, neurologic complications, and select neurologic complications was significantly higher for piecemeal resection in eloquent brain compared to en bloc resection; however, there was not a difference in 1-year mortality or major neurologic complications. |
| Quigley et al,7 2015 |
Retrospective analysis of 162 consecutive patients with oligometastatic disease who underwent surgery + SRS boost (49 patients) or SRS alone (113 patients). Patients who received prior WBRT were excluded. |
III |
RPA class was statistically different between groups. The surgery + SRS group had larger maximal tumor dimension, larger treatment volume, lower average radiation dose to tumor margin, and initial tumor volume. Median survival for complete resection + SRS vs incomplete resection + SRS vs SRS alone was 14.1 months, 7.1 months, and 6.9 months respectively (p = .032). Overall survival was associated with complete surgical resection (HR = 0.55, p = .01), age (HR = 1.21/decade, p = . 37), and ECOG score (HR = 1.9, p =. 01). Time to local recurrence was associated with radiation-sensitive pathology (HR = 0.34, p = .001), treatment volume (HR = 1.078/mL, p = .002), and complete tumor resection (HR = 0.37, p = .015). Incomplete tumor resection and SRS alone had equivalent time to local recurrence and median survival. Using propensity score matching ad Cox regression demonstrated that complete resection was a significant factor in survival (HR = 0.52, p = .03) |
| Wang et al,20 2015 |
Retrospective analysis of 528 patients undergoing treatment for one or multiple brain metastases among various histologies. Treatment included SRS alone (206 patients), SRS + WBRT (111 patients), surgery + SRS (109 patients), surgery + SRS + WBRT (102 patients). |
III |
On univariate analysis, patients treated with surgery + SRS (HR = 0.468, p < .001), SRS + WBRT (HR = 0.636, p = .001), or surgery + SRS+WBRT (HR = 0.481, p < .001) all had improved overall survival compared with SRS alone. Multivariate analysis confirmed that surgery + SRS + WBRT had the longest survival (HR = 0.467, p < .001) compared with SRS alone but was equivalent to the other bimodality approaches. Surgery + SRS without WBRT did not adversely affect survival. Predictors of survival on multivariate analysis included uncontrolled primary extra-CNS disease, age, and KPS. |
| Johnson et al,12 2016 |
Single institution retrospective analysis of 330 patients treated with radiosurgery for intact (218 patients) or resected metastases (112 patients). |
III |
Differences between groups were notable for age, RPA class, and total tumor volume. The 1-year cumulative incidence of LMD was 5.2% for SRS alone, compared with 16.9% for surgery + SRS (p < .01). Univariate analysis of the surgical patients did not reveal predictors of LMD, including en bloc resection or subtotal resection. On multivariate analysis, prior surgery and breast cancer were significant predictors of LMD (p < .01 and p = .03, respectively). There was a trend toward increased median overall survival for surgery vs SRS alone (12.9 vs 10.6 months, p = .06) |
| Arita et al,14 2014 |
Retrospective analysis of 264 surgical cases for various brain metastases to evaluate clinical characteristics that were predictive of early death after surgery (within 6 months). |
III |
A total of 23% of patients died within 6 months of surgery. On multivariate analysis, factors associated with early death include a decrease in postoperative KPS (<70) (p = .041), lack of postoperative systemic therapy (p < .0001), and uncontrolled extracranial disease (p = .0022). Preoperative KPS <70, pre- and postoperative RPA class were only associated with early death in univariate analysis. |
| Baykara et al,6 2014 |
Single institution retrospective study of 138 patients undergoing treatment for metastatic non–small cell lung cancer. Treatment groups consisted of 44.2% receiving SRS, 24.6% SRS + WBRT, 10.8% surgery + WBRT, 12.3% WBRT. Patients had 1-4 intracranial metastases. |
III |
Local failure relapse-free survival for surgery + WBRT was significantly higher than WBRT alone (p < .0001). By univariate analysis, overall survival was significantly longer for surgery + WBRT compared to other treatment groups (p = .037). Median survival was significantly longer for surgery + WBRT compared with either WBRT alone (29.6 vs 16.7 months, p = .006) or SRS + WBRT (9.3 months, p = .007). |
| Obermueller et al,26 2014 |
Retrospective analysis of 206 brain metastases that underwent surgery. A total of 56 patients had tumor involvement in eloquent motor areas while 150 were in noneloquent areas. |
III |
Cases with gross total resection had overall survival of 9.1 months compared with 7.5 months with subtotal resection (p = .08). There was no association between postoperative impairment in motor function and tumor histology. For surgery in eloquent motor cortex, there was a trend toward postoperative paresis (p = .101). Among patients with surgery in eloquent cortex, high RPA class was associated with postoperative paresis (p < .05). A similar finding was observed for surgery in noneloquent cortex (p < .001) as well. Prior treatment with radiation in the motor eloquent group led to a new postoperative deficit in 55% of patients, compared with 13% who did not have preoperative radiation (p = .01). In nonmotor eloquent group, prior treatment with radiation led to a new deficit in 28.1% of cases, compared with 14% in patients who did not have preoperative radiation (p < .05). In both groups, preoperative chemotherapy was not associated with postoperative deficits. |
| Ojerholm et al,32 2014 |
Retrospective analysis of 91 patients without prior WBRT who received SRS to 96 resection cavities across multiple tumor histologies. |
III |
On multivariate analysis, preoperative metastases diameter >3 cm and residual or recurrent tumor at the time of SRS was associated with local failure (p = .04 and .008, respectively). Leptomeningeal carcinomatosis was associated with breast histology and infratentorial cavities (p = .024 and .012, respectively). |
| Kim et al,8 2013 |
Retrospective analysis of 27 patients undergoing SRS and 11 patients treated surgically for colorectal brain metastases. |
III |
The surgical group had a significant improvement in local control compared with SRS (90% vs 71%, p = .006), symptom relief at 3 months (72% vs 18%, p = .005), and median overall survival (16.2 vs 5.6 months, p = .0035). In multivariate analysis, controlled primary tumor and solitary metastases were associated with prolonged overall survival (p = .038 and p = .024, respectively). Surgery was associated with longer local control (p = .034). Of note, the surgical population was significantly younger than the SRS population (56 vs 66, p = .014), treated tumors >3 cm (81% vs 7.4%, p < .001), and treated solitary tumors (100% vs 37%, p < .001). |
| Lee et al,31 2013 |
Retrospective 17-year longitudinal study of 157 patients undergoing surgery for various histologic brain metastases. A total of 69.4% of patients underwent adjuvant WBRT while 10.8% of patients underwent SRS. |
III |
The median survival after gross total resection was 20.4 months compared with 15.1 months after subtotal resection (p = .016). Patients with stable primary extracranial disease and RPA class I had longer overall survival (p = .032, p = .022). Among patients in the RPA class I, gross total resection led to a significant increase in overall survival compared to subtotal resection (p = .022). Adjuvant treatment did not lead to an improvement in survival or clinical outcome. |
| Miller et al,23 2013 |
Single institutional retrospective analysis of 34 patients with metastatic melanoma brain metastases. Among the patients, 22 had a single metastasis while 12 patients had two or more lesions. |
III |
Patients with single brain metastasis had a median survival of 13 months compared with 5.0 months for patients with two or more metastases (p = .014). Patients who did not receive adjuvant therapy after surgery lived significantly shorter than patients receiving postoperative radiation, chemotherapy, or immunotherapy (2 months vs 6 months, p = .014). Patients with isolated intracerebral relapse survived significantly longer than patients with systemic progression (6 months vs 3 months, p = .003). Patients receiving local therapy consisting of surgery or SRS for recurrence had improved survival compared to recurrence treated with WBRT, chemotherapy, or supportive therapy (6 months vs 3 months, p = .011). Patients with high performance status had prolonged median survival (7 months vs 1 month, p = .001). The only postoperative adjuvant treatment associated with improved overall survival was immunotherapy with interferon therapy (50 months vs 7 months, p = .039); however, only 3 patients were included in the immunotherapy cohort, and the authors caution that these patients may represent a selection bias towards patients with better prognosis. |
| Rades et al,13 2012 |
Matched pair analysis comparing WBRT + radiosurgery (46 patients) compared to surgery + WBRT + boost (46 patients) for single brain metastasis. |
II |
No significant difference was observed for 1-year local control, 1-year intracerebral control, and 1-year survival. On univariate analysis, improved survival was associated with KPS >70 (p = .032), absence of extracerebral metastases (p = .003), RPA class I (p = .014), and GPA 3.0-4.0 (p = .01). |
| Rades et al,15 2012 |
Retrospective analysis of 41 patients treated with WBRT + radiosurgery compared to 111 patients treated with surgery + WBRT for a single brain metastasis. |
III |
A significant difference in 1-year local control was observed between WBRT + radiosurgery (87%) compared to surgery + WBRT (56%) (p = .01). Using a Cox proportional hazards model, treated regimen remained significant (2.46, p = .005). Difference in treatment did not result in a significant difference in overall survival. On multivariate analysis, independent factors associated with overall survival included KPS, extracerebral metastases, RPA class, and GPA. |
| d’Agostino et al,17 2011 |
Retrospective analysis of patients with brain metastases undergoing surgery + WBRT (50 patients) compared to WBRT + SRS (47 patients). |
III |
No statistically significant difference was observed in local control or overall survival at 1 or 5 years. Groups were matched for WBRT schedule, age, gender, performance status, tumor type, number of metastases (<3) but did not appear matched for tumor size. Notably, survival was not associated with RPA class, primary tumor, or number of brain lesions. |
| Elaimy et al,10 2011 |
Retrospective single institution study of 275 patients treated WBRT (117 patients), SRS (65 patients), WBRT + SRS (48 patients), surgery + SRS (15 patients), surgery + WBRT (11 patients), surgery + WBRT + SRS (19 patients). |
III |
On multivariate analysis, improved survival was associated with SRS compared to WBRT alone (p < .001), surgery + SRS compared to SRS alone (p = .02), non–small cell lung cancer compared to melanoma or renal cell carcinoma (p < .001), and patients with breast cancer when compared to non–small cell lung cancer (p < .001). There was no association with survival and number of brain metastases or tumor volume. |
| Jung et al,5 2011 |
Retrospective analysis of 126 patients with varying number of colorectal cancer brain metastases treated at a single institution. Treatment included steroids alone (20 patients), WBRT (45 patients), SRS (41 patients) and surgery + radiation (20 patients). |
III |
Among the four treatment modalities, surgical patients had the longest median survival (11.5 months, p < .001). However, the authors did not state whether median survival for steroids (1.5 months), WBRT (4 months), or SRS (9.5 months) were significant. Multivariate analysis demonstrated that RPA class and amount of chemotherapy prior to brain metastases was associated with survival. |
| Marko et al,11 2011 |
Retrospective single institution study examining 26 patients with incidentally found non-small cell lung cancer brain metastases treated with upfront SRS alone compared to patients treated with WBRT (121 patients), WBRT + surgery (45 patients), or WBRT + SRS (15 patients). Inclusion criteria included KPS > 90, minimal neurologic symptoms, and SRS treatment within 60 days of diagnosis of the metastasis. |
III |
Survival among patients treated with SRS was not statistically different from comparable patients treated with WBRT or WBRT + SRS. Although not statistically significant, there was a trend towards improved mean survival in patients treated with WBRT + surgery compared to SRS alone (20.1 months vs 12.3 months, p = .07). Of note, a comparison between SRS alone and surgery + SRS was lacking. |
| Stark et al,22 2011 |
Retrospective analysis of 309 patients who underwent surgery for newly diagnosed brain metastases |
III |
Factors associated with survival on univariate analysis included age, extracranial metastases, preoperative KPS >70, complete resection based on postoperative imaging, postoperative KPS >70, radiotherapy, and re-craniotomy for recurrence. Multivariate analysis demonstrated age (above or below 65), postoperative KPS (above or below 70), extracranial metastases, radiotherapy, and re-craniotomy for recurrence as independent factors associated with prolonged survival. Further analysis was performed using an age threshold of 65 years to stratify patient prognosis. Among patients <65, extracranial metastases, preoperative KPS (above or below 70), complete resection, radiotherapy, and recraniotomy for recurrence were identified as independent prognostic factors. |
| Hassaneen et al,28 2010 |
Retrospective analysis of 29 patients undergoing craniotomy for lateral ventricle metastases. |
III |
Factors associated with improved survival on univariate analysis include KPS <80, single intracranial metastasis, renal cell carcinoma, and resection method (en bloc rather than piecemeal). Associations with survival time on multivariate analysis included KPS >80, primary RCC, and en bloc resection. |
| Jagannathan et al,25 2010 |
Retrospective analysis of 912 patients who failed gamma knife radiation for intracranial metastases. A total of 15 patients underwent surgical resection following gamma knife. |
III |
Mean survival for patients in whom viable tumor was identified was significantly lower than for patients in whom only necrosis was seen (9.4 vs 15.1 months, p < .05). |
| Kalani et al,16 2010 |
Retrospective analysis of 150 patients who underwent resection of solitary brain metastasis and SRS. |
III |
Patients with a pretreatment KPS of ≥90 had median survival of 23.2 months compared to patients with a pretreatment KPS |
| Patel et al,30 2010 |
Retrospective analysis to examine factors influencing local recurrence in 570 cases who underwent surgery of a previously untreated single brain metastasis. |
III |
Histology of primary cancer was not predictive of local recurrence. Univariate analysis demonstrated an association for local recurrence with piecemeal resection vs en bloc resection (of 1.7, p = .03) and tumors >9.7cm3 (HR 1.7, p = .02). On multivariate analysis, en bloc resection was associated with decreased rate of local recurrence for tumors < 9.71cm3. Of note, the median volume of tumors resected by piecemeal was 15.87 cm3 compared with 7.59 cm3 for en bloc. |
| Aprile et al,18 2009 |
Retrospective analysis of 30 patients with colorectal cancer brain metastases undergoing surgery (14 patients) vs surgery + WBRT (16 patients). |
III |
Patients with surgery + WBRT had median survival of 7.6 months vs 4.7 months for surgery alone (p = .014). On multivariate analysis, WBRT was associated with improved overall survival. Of note, statistical analysis of baseline patient population is lacking. Authors conclude that aggressive treatment is warranted in patients with adequate functional status and controlled systemic disease. |
| Kano et al,24 2009 |
Retrospective analysis of 58 patients undergoing SRS followed by surgery for brain metastases. |
III |
On univariate analysis, factors associated with patient survival included preoperative RPA classification, KPS >70, systemic disease status, and the interval between SRS and resection (8.8 months for surgery ≥3 months after SRS vs 5.8 months for surgery 3 months) were best candidates for surgery while RPA class and systemic disease status should also be considered. |
| Lindvall et al,4 2009 |
Retrospective study of the treatment of solitary brain metastases with surgery + WBRT (59 patients) vs hypofractionated stereotactic irradiation (HCSRT) (47 patients). |
III |
The overall median survival for surgery + WBRT was 7.9 months vs 5.0 months for HCSRT (p = .014). For patients with tumor volume |
| Suki et al,27 2009 |
Retrospective analysis of leptomeningeal disease (LMD) in patients with supratentorial brain metastases undergoing SRS (285 patients), piecemeal (191 patients) or en bloc (351 patients) resection. |
III |
Risk of LMD was significantly higher with piecemeal resection compared to SRS (HR = 5.8, p = .002) and en bloc resection (HR = 2.7, p = .009). Melanoma was most susceptible to LMD comparing piecemeal vs en bloc (HR = 8.4, p = .007). There was no difference in LMD between en bloc resection and SRS. Additional multivariate predictors of LMD included tumor functional grade III and pre-procedure tumor volume >9.6 cc. |
| Yoo et al,33 2009 |
Retrospective analysis of patients undergoing microscopic total resections (tumor resection with additional removal of ~5 mm of normal-appearing brain tissue; MTR) in noneloquent areas (43 patients) compared with patients undergoing gross total resections (GTR) in eloquent locations (51 patients). |
III |
MTR led to improved local control compared to GTR (local recurrence of 23.3% vs 43.1%, p = .04). Multivariate analysis demonstrated an association of decreased local recurrence with MTR and postoperative radiotherapy. Extent of surgery was not associated with overall survival on univariate or multivariate analysis. Of note, 37% of GTR patients had KPS |
| Rades et al,21 2009 |
Matched-pair analysis of patients with 1 or 2 brain metastases undergoing WBRT + SRS (47 patients) compared to surgery + WBRT + boost to the operative (47 patients) |
II |
Median survival for surgery + WBRT + boost was 25 months compared to 15 months for WBRT + SRS. However, these results were not statistically significant (p = .19). In addition to lack of a statistically significant difference in 1-year survival, there was no different in 1-year intracerebral control rate or 1-year local control rate. On multivariate analysis, improved survival was associated with performance status, lack of extracerebral metastases, RPA class I, and interval from tumor diagnosis to WBRT. |
| Muacevic et al,19 2009 |
Phase III multicenter trial comparing treatment with gamma knife (31 patients) to surgery + WBRT (33 patients). Patients ranged from 18-80 years of age, had a single brain metastasis ≤3 cm in size, KPS ≥ 0, and stable systemic disease. Primary endpoint was overall survival. Secondary endpoints were recurrence of tumor in the brain, health-related quality of life, and treatment-related toxicity. |
II |
Radiosurgery was associated with higher rates of distant recurrence, but difference was lost after adjusting for effects of salvage radiosurgery. No difference in overall survival, neurologic death rate, or local recurrence. Radiosurgery was associated with a shorter hospital stay, faster steroid taper, and lower rate of grade 1 or 2 toxicities. Quality of life was improved at 6 weeks’ postradiosurgery but lost after 6 months. Radiosurgery compared with surgery + WBRT yielded similar results, except for distant tumor control but could potentially be addressed by salvage radiation. |
| Ogawa et al,2 2008 |
Retrospective analysis of 65 patients with breast cancer brain metastases. 11 patients underwent surgery followed by radiotherapy while 54 patients were treated by radiotherapy alone. |
III |
Univariate and multivariate analysis demonstrated an improvement in 1-year overall survival and brain metastases progression/recurrence-free survival for patients with KPS ≥70, surgery + radiotherapy (73% vs 19% 1-year overall survival), and chemotherapy following radiotherapy. |
| Rades et al,3 2008 |
Retrospective analysis of 195 patients with single brain metastases treated with surgery followed by WBRT (99 patients) compared to WBRT alone (96 patients). |
III |
Median survival for surgery + WBRT was 11.5 months compared with 8 months for WBRT alone (p < .001). On multivariate analysis, surgery was associated with improved overall survival, local control, and control within the entire brain but not with improved distant intracranial control. |
CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; GTR, gross total resection; HCSRT, hypofractionated stereotactic irradiation; HR, hazard ratio; KPS, Karnofsky Performance Status; LMD, leptomeningeal disease; MTR, microscopic total resection; RCC, renal cell carcinoma; RPA, recursive partitioning analysis; SRS, stereotactic radiosurgery; WBRT, whole brain radiation therapy.
Appendix A: Primary Search Strategies
OVID MEDLINE, searched on Aug 9, 2016:
1. brain neoplasms/
2. brain neoplasms/su
3. (brain neoplasm$ or brain tumor$ or brain tumour$ or brain cancer or brain lesion$).ti,ab.
4. (surgery or surgical or operative or resect$).ti,ab.
5. Neoplasm Metastasis/
6. (Metastasis or Metastases or metastatic or metastasize$ or metastasise$).ti,ab.
7. 1 and 4 and (5 or 6)
8. 2 and (5 or 6)
9. 3 and 4 and (5 or 6)
10. 7 or 8 or 9
11. age-18-and-under/
12. (pediatr$ or paediatr$ or child$ or infan$ or adolesc$).ti,ab,hw,jn,jw,de.
13. 11 or 12
14. 10 not 13
15. (brain or surgery or surgical or operative or resect$ or metas$).ti.
16. 14 and 15
17. ("more than 1" or "1 or more" or multiple).ti,ab.
18. (case report$ or comment or editorial or letter or news or patient education handout or portraits).pt,ti.
19. 16 not 18
20. limit 19 to (english language and yr="2008 - 2015")
21. 17 and 20
22. 20 or 21
PUBMED (NLM), searched on August 17, 2016:
(((Metastasis[Title] OR Metastases[Title] OR metastatic[Title] OR metastasize*[Title] OR metastasise*[Title])) AND (surgery[Title] OR surgical[Title] OR operative[Title] OR resect*[Title])) AND brain[Title]
OR
((((Metastasis[Title] OR Metastases[Title] OR metastatic[Title] OR metastasize*[Title] OR metastasise*[Title])) AND (surgery[Title] OR surgical[Title] OR operative[Title] OR resect*[Title]))) AND Brain Neoplasms [Majr]
NOT: ((case report*[Publication Type] OR comment[Publication Type] OR editorial[Publication Type] OR letter[Publication Type] OR news[Publication Type] OR patient education handout[Publication Type] OR portraits[Publication Type])) OR (case report*[Title] OR comment[Title] OR editorial[Title] OR letter[Title] OR news[Title] OR patient education handout[Title] OR portraits[Title]
(multiple[Title/Abstract] OR "more than 1"[Title/Abstract])
Filters: Publication date from 2008/01/01 to 2015/12/31; Humans; English; Adult: 19+ years
Total: 1060 results
REFERENCES
- Kalkanis SN, Kondziolka D, Gaspar LE, et al. The role of surgical resection in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. Jan 2010;96(1):33-43.
- Ogawa K, Yoshii Y, Nishimaki T, et al. Treatment and prognosis of brain metastases from breast cancer. J Neurooncol. Jan 2008;86(2):231-238.
- Rades D, Kieckebusch S, Haatanen T, Lohynska R, Dunst J, Schild SE. Surgical resection followed by whole brain radiotherapy versus whole brain radiotherapy alone for single brain metastasis. Int J Radiat Oncol Biol Phys. Apr 1 2008;70(5):1319-1324.
- Lindvall P, Bergstrom P, Lofroth PO, Tommy Bergenheim A. A comparison between surgical resection in combination with WBRT or hypofractionated stereotactic irradiation in the treatment of solitary brain metastases. Acta Neurochir (Wien). Sep 2009;151(9):1053-1059.
- Jung M, Ahn JB, Chang JH, et al. Brain metastases from colorectal carcinoma: prognostic factors and outcome. J Neurooncol. Jan 2011;101(1):49-55.
- Baykara M, Kurt G, Buyukberber S, et al. Management of brain metastases from non-small cell lung cancer. J Cancer Res Ther. Oct-Dec 2014;10(4):915-921.
- Quigley MR, Bello N, Jho D, Fuhrer R, Karlovits S, Buchinsky FJ. Estimating the additive benefit of surgical excision to stereotactic radiosurgery in the management of metastatic brain disease. Neurosurgery. Jun 2015;76(6):707-712; discussion 712-703.
- Kim HJ, Huh JW, Jung TY, et al. Clinical outcome with gamma-knife surgery or surgery for brain metastases from colorectal cancer. J Clin Neurosci. Oct 2013;20(10):1417-1421.
- Bougie E, Masson-Cote L, Mathieu D. Comparison Between Surgical Resection and Stereotactic Radiosurgery in Patients with a Single Brain Metastasis from Non-Small Cell Lung Cancer. World Neurosurg. Jun 2015;83(6):900-906.
- Elaimy AL, Mackay AR, Lamoreaux WT, et al. Multimodality treatment of brain metastases: an institutional survival analysis of 275 patients. World J Surg Oncol. Jul 5 2011;9:69.
- Marko NF, Suh JH, Chao ST, et al. Gamma knife stereotactic radiosurgery for the management of incidentally-identified brain metastasis from non-small cell lung cancer. J Neurooncol. Sep 2011;104(3):817-824.
- Johnson MD, Avkshtol V, Baschnagel AM, et al. Surgical Resection of Brain Metastases and the Risk of Leptomeningeal Recurrence in Patients Treated With Stereotactic Radiosurgery. Int J Radiat Oncol Biol Phys. Mar 1 2016;94(3):537-543.
- Rades D, Kueter JD, Meyners T, et al. Single brain metastasis: resection followed by whole-brain irradiation and a boost to the metastatic site compared to whole-brain irradiation plus radiosurgery. Clin Neurol Neurosurg. May 2012;114(4):326-330.
- Arita H, Narita Y, Miyakita Y, Ohno M, Sumi M, Shibui S. Risk factors for early death after surgery in patients with brain metastases: reevaluation of the indications for and role of surgery. J Neurooncol. Jan 2014;116(1):145-152.
- Rades D, Veninga T, Hornung D, Wittkugel O, Schild SE, Gliemroth J. Single brain metastasis: whole-brain irradiation plus either radiosurgery or neurosurgical resection. Cancer. Feb 15 2012;118(4):1138-1144.
- Kalani MY, Filippidis AS, Kalani MA, et al. Gamma Knife surgery combined with resection for treatment of a single brain metastasis: preliminary results. J Neurosurg. Dec 2010;113 Suppl:90-96.
- D'Agostino GR, Autorino R, Pompucci A, et al. Whole-brain radiotherapy combined with surgery or stereotactic radiotherapy in patients with brain oligometastases: long-term analysis. Strahlenther Onkol. Jul 2011;187(7):421-425.
- Aprile G, Zanon E, Tuniz F, et al. Neurosurgical management and postoperative whole-brain radiotherapy for colorectal cancer patients with symptomatic brain metastases. J Cancer Res Clin Oncol. Mar 2009;135(3):451-457.
- Muacevic A, Wowra B, Siefert A, Tonn JC, Steiger HJ, Kreth FW. Microsurgery plus whole brain irradiation versus Gamma Knife surgery alone for treatment of single metastases to the brain: a randomized controlled multicentre phase III trial. J Neurooncol. May 2008;87(3):299-307.
- Wang TJ, Saad S, Qureshi YH, et al. Outcomes of gamma knife radiosurgery, bi-modality & tri-modality treatment regimens for patients with one or multiple brain metastases: the Columbia University Medical Center experience. J Neurooncol. Apr 2015;122(2):399-408.
- Rades D, Kueter JD, Pluemer A, Veninga T, Schild SE. A matched-pair analysis comparing whole-brain radiotherapy plus stereotactic radiosurgery versus surgery plus whole-brain radiotherapy and a boost to the metastatic site for one or two brain metastases. Int J Radiat Oncol Biol Phys. Mar 15 2009;73(4):1077-1081.
- Stark AM, Stohring C, Hedderich J, Held-Feindt J, Mehdorn HM. Surgical treatment for brain metastases: Prognostic factors and survival in 309 patients with regard to patient age. J Clin Neurosci. Jan 2011;18(1):34-38.
- Miller D, Zappala V, El Hindy N, et al. Intracerebral metastases of malignant melanoma and their recurrences--a clinical analysis. Clin Neurol Neurosurg. Sep 2013;115(9):1721-1728.
- Kano H, Kondziolka D, Zorro O, Lobato-Polo J, Flickinger JC, Lunsford LD. The results of resection after stereotactic radiosurgery for brain metastases. J Neurosurg. Oct 2009;111(4):825-831.
- Jagannathan J, Bourne TD, Schlesinger D, et al. Clinical and pathological characteristics of brain metastasis resected after failed radiosurgery. Neurosurgery. Jan 2010;66(1):208-217.
- Obermueller T, Schaeffner M, Gerhardt J, Meyer B, Ringel F, Krieg SM. Risks of postoperative paresis in motor eloquently and non-eloquently located brain metastases. BMC Cancer. Jan 14 2014;14:21.
- Suki D, Hatiboglu MA, Patel AJ, et al. Comparative risk of leptomeningeal dissemination of cancer after surgery or stereotactic radiosurgery for a single supratentorial solid tumor metastasis. Neurosurgery. Apr 2009;64(4):664-674; discussion 674-666.
- Hassaneen W, Suki D, Salaskar AL, et al. Surgical management of lateral-ventricle metastases: report of 29 cases in a single-institution experience. J Neurosurg. May 2010;112(5):1046-1055.
- Patel AJ, Suki D, Hatiboglu MA, Rao VY, Fox BD, Sawaya R. Impact of surgical methodology on the complication rate and functional outcome of patients with a single brain metastasis. J Neurosurg. May 2015;122(5):1132-1143.
- Patel AJ, Suki D, Hatiboglu MA, et al. Factors influencing the risk of local recurrence after resection of a single brain metastasis. J Neurosurg. Aug 2010;113(2):181-189.
- Lee CH, Kim DG, Kim JW, et al. The role of surgical resection in the management of brain metastasis: a 17-year longitudinal study. Acta Neurochir (Wien). Mar 2013;155(3):389-397.
- Ojerholm E, Lee JY, Thawani JP, et al. Stereotactic radiosurgery to the resection bed for intracranial metastases and risk of leptomeningeal carcinomatosis. J Neurosurg. Dec 2014;121 Suppl:75-83.
- Yoo H, Kim YZ, Nam BH, et al. Reduced local recurrence of a single brain metastasis through microscopic total resection. J Neurosurg. Apr 2009;110(4):730-736.
- Lonser RR, Song DK, Klapper J, et al. Surgical management of melanoma brain metastases in patients treated with immunotherapy. J Neurosurg. Jul 2011;115(1):30-36.
Source: Neurosurgery, January 9, 2019