Guidelines on the Management of Patients with Vestibular Schwannoma
9. Emerging Therapies for the Treatment of Patients with Vestibular Schwannomas
download pdf Neurosurgery, 2017
Sponsored by: Congress of Neurological Surgeons (CNS) and the AANS/CNS Tumor Section
Endorsed by: Joint Guidelines Committee of the American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons (CNS)
Authors:
Jamie J. Van Gompel, MD1,3, Siviero Agazzi, MD, MBA2, Matthew L. Carlson, MD1,3, Dare A. Adewumi, MD4, Constantinos G. Hadjipanayis, MD, PhD5, Joon H. Uhm, MD6, Jeffrey J. Olson, MD7
1. Department of Neurosurgery, Mayo Clinic, Rochester, Minnesota, USA
2. Department of Neurosurgery and Brain Repair, College of Medicine, University of South Florida, Tampa, Florida, USA
3. Department of Otorhinolaryngology, Mayo Clinic, Rochester, Minnesota, USA
4. The Greater Houston Neurosurgery Center, The Woodlands, Texas, USA
5. Department of Neurosurgery, Mount Sinai Beth Israel, Icahn School of Medicine at Mount Sinai, New York, New York
6. Department of Neurology and Department of Oncology, Mayo Clinic, Rochester, Minnesota, USA
7. Department of Neurosurgery, Emory University School of Medicine, Atlanta, Georgia
Correspondence:
Jamie J. Van Gompel, MD
Department of Neurosurgery
Mayo Clinic, 200 First Street SW
Rochester, Minnesota 55905
Telephone: 507-284-2511; Fax: 507-284-5206
Email: vangompel.jamie@mayo.edu
Keywords: Acoustic neuroma, emerging therapies, endoscope, novel drug therapies, vestibular schwannoma
No part of this manuscript has been published or submitted for publication elsewhere.
Abbreviations
CSF: Cerebrospinal fluid
NF2: Neurofibromatosis 2
VS: Vestibular schwannoma
Abstract
Medical Therapy
Question
What is the role of bevacizumab in the treatment of patients with vestibular schwannomas (VSs)?
Target Population
Adults with histologically proven or suspected VSs with neurofibromatosis type 2 (NF2).
Recommendations
Level 3: It is recommended that bevacizumab be administered to radiographically reduce the size or prolong tumor stability in patients with NF2 without surgical options.
Level 3: It is recommended bevacizumab be administered to improve hearing or prolong time to hearing loss in patients with NF2 without surgical options.
Question
Is there a role for lapatinib, erlotinib, or everolimus in the treatment of patients with VSs?
Target Population
Adults with histologically proven or suspected VSs and NF2
Recommendation
Level 3: Lapatinib may be considered for use in reducing VS size and improvement in hearing in NF2.
Level 3: Erlotinib is not recommended for use in reducing VS size or improvement in hearing in patients with NF2.
Level 3: Everolimus is not recommended for use in reducing VS size or improvement in hearing in NF2.
Question
What is the role of aspirin, to augment inflammatory response, in the treatment of patients with VSs?
Target Population
Any patient with a VS undergoing observation
Recommendation
Level 3: It is recommended that aspirin administration may be considered for use in patients undergoing observation of their VSs.
Question
Is there a role for treatment of vasospasm, ie, nimodipine or hydroxyethyl starch, perioperatively to improve facial nerve outcomes in patients with VSs?
Target Population
Adults with histologically proven or suspected VSs
Recommendation
Level 3: Perioperative treatment with nimodipine (or with addition of hydroxyethyl starch) should be considered to improve postoperative facial nerve outcomes and may improve hearing outcomes.
Prehabilitation
Question
Is there a role for preoperative vestibular rehab or vestibular ablation with gentamicin for patients surgically treated for VSs?
Target Population
Adults with histologically proven or suspected VSs
Recommendations
Level 3: Preoperative vestibular rehabilitation is recommended to aid in postoperative mobility after VS surgery.
Level 3: Preoperative gentamicin ablation of the vestibular apparatus should be considered to improve postoperative mobility after VS surgery.
Surgical Therapy
Question
Does endoscopic assistance make a difference in resection or outcomes in patients with VSs?
Target population
VS patients, who are surgical candidates. Inclusion in this analysis required resection utilizing the endoscope, either as the primary operative visualization or microscopic assistance with more than 20 patients treated.
Recommendation
Endoscopic assistance is a surgical technique that the surgeon may choose to use in order to aid in visualization.
Introduction
Rationale
Successful VS treatment has not been achieved uniformly and neurologic surgeons continue to strive to improve treatment of these tumors. Fortunately, there is research proceeding on several fronts to improve treatment of this highly specialized tumor. To provide insight into these exciting areas, several treatments will be presented in this guideline that are relevant to VS treatment.
Objectives
The objectives of this paper are to assess both comparative and noncomparative studies of emerging therapies for VSs. Questions about the following treatments were considered (notably, other therapies, such as proton beam therapy, were considered; however, they were part of the preceding guidelines papers and therefore removed from this guideline preparation):
1. Medical
- What is the role of bevacizumab in the treatment of patients with VSs?
- What is the role of AR42, a histone deacetylase inhibitor, in the treatment of patients with VS?
- Is there a role for imatinib mesylate, lapatinib, erlotinib, or everolimus in the treatment of patients with VSs?
- What is the role of aspirin, to augment inflammatory response, in the treatment of patients with VSs?
- Is there a role for treatment of vasospasm, ie, nimodipine or hydroxyethyl starch, perioperatively to improve facial nerve outcomes in patients with VSs?
2. Prehabilitation
- Is there a role for preoperative vestibular rehab or vestibular ablation with gentamicin for patients surgically treated for VSs?
3.Surgical
- Does endoscopic assistance make a difference in resection or outcomes in patients with VSs?
Methods
Writing Group and Question Establishment
The evidence-based clinical practice guideline taskforce members and the Joint Tumor Section of the American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons (CNS) have prioritized an update of the guidelines for management of VSs. A series of writers were identified and screened for conflict of interest. This group in turn agreed on a set of questions addressing the topic at hand and conducted a systematic review of the literature relevant to the use of emerging therapies in patients with sporadic VSs. Additional details of the systematic review are provided below and within the introduction and methodology chapter of the guideline (here).
Search Strategies
The task force collaborated with a medical librarian to search for articles published between January 1, 1966 and December 31, 2014. Two electronic databases, PubMed and the Cochrane Central Register of Controlled Trials, were searched. Strategies for searching electronic databases were constructed by the evidence-based clinical practice guideline taskforce members and the medical librarian using previously published search strategies to identify relevant studies.1–8
The task force supplemented searches of electronic databases with manual screening of the bibliographies of all retrieved publications. The task force also searched the bibliographies of recent systematic reviews and other review articles for potentially relevant citations. All articles identified were subject to the study selection criteria listed below.
The task force made every effort to obtain a complete set of relevant articles to ensure the guideline is not based on a biased subset of articles. The specific search strategies for each question can be found below.
For searches, PubMed was used for medical therapies. The root search was as follows: Neuroma, Acoustic [MeSH], (vestibular [Title/Abstract] OR vestibulocochlear [Title/Abstract] OR acoustic [Title/Abstract]) AND (neuroma* [Title/Abstract] OR neurilemmoma* [Title/Abstract] OR neurilemoma* [Title/Abstract] OR neurinoma* [Title/Abstract] OR tumor* [Title/Abstract] OR tumour* [Title/Abstract] OR schwannoma* [Title/Abstract]), #1 OR #2. Topic Specific search was performed using the following root search: Antineoplastic Agents [Mesh] OR Antineoplastic Agents [NM] OR Avastin [TIAB] OR Bevacizumab [NM] OR Bevacizumab [TIAB] OR Angiogenesis inhibitors [Mesh] OR Angiogenesis inhibitors [PA] OR Angiogenesis inhibitors [NM] OR Antibodies, Monoclonal, Humanized [Mesh] OR Vascular Endothelial Growth Factor A/antagonists and inhibitors [MH] OR AR42 [TIAB] OR AR 42 [TIAB] OR Histone deacetylase inhibitors [Mesh] OR Histone deacetylase inhibitors [NM] OR Aspirin [Mesh] OR Aspirin [TIAB] OR Nimodipine [Mesh] OR Nimodipine [TIAB] OR Hydroxyethyl starch [TIAB] OR lapatinib [TIAB] OR lapatinib [NM] OR Everolimus [TIAB] OR Everolimus [NM] OR Afinitor [TIAB] OR Axitinib [TIAB] OR Axitinib[NM] OR Nilotinib [TIAB] OR Molecular Targeted Therapy [MH] OR Drug therapy [sh] OR administration and dosage [SH] OR Antagonists and inhibitors [SH] OR Pharmacotherapy [TIAB] OR ((emerging [TIAB] OR emergent [TIAB] OR molecular [TIAB] OR gene [TIAB] OR drug [TIAB] OR targeted [TIAB] OR systemic [TIAB]) AND (therapy [TIAB] OR therapies [TIAB] OR treatment* [TIAB])) , #1 AND #2, (animal [MeSH] NOT human [MeSH]) OR cadaver [MeSH] OR cadaver* [Titl] OR comment [PT] OR letter [PT] OR editorial [PT] OR addresses [PT] OR news [PT] OR “newspaper article” [PT] OR case reports [PT] OR case report [Title]. In addition, a Cochrane Central Search was performed utilizing: MeSH descriptor: [Neuroma, Acoustic]: explode all trees, ((vestibular or vestibulocochlear or acoustic) and (neuroma* or neurilemmoma* or neurilemoma* or neurinoma* or tumor* or schwannoma*)):ti,ab,kw, #1 or #2, MeSH descriptor: [Antineoplastic Agents] explode all trees, MeSH descriptor: [Angiogenesis inhibitors] explode all trees, MeSH descriptor: [Antibodies, Monoclonal, Humanized] explode all trees, MeSH descriptor: [Vascular endothelial growth factor A/antagonists and inhibitors] explode all trees, MeSH descriptor: [Histone deacetylase inhibitors] explode all trees, MeSH descriptor: [Aspirin] explode all trees, MeSH descriptor: [Nimodipine] explode all trees, MeSH descriptor: [Molecular Targeted therapy], Any MeSH descriptor with qualifier(s): [Drug therapy], Any MeSH descriptor with qualifier(s): [Administration and dosage], Any MeSH descriptor with qualifier(s): [Antagonists and inhibitors], (Avastin or bevacizumab or AR42 or AR 42 or aspirin or nimodipine or hydroxyethyl starch or lapatinib or everolimus or afinitor or axitinib or nilotinib or pharmacotherapy):ti,ab,kw , ((emerging or emergent or molecular or gene or drug or targeted or systemic) and (therapy or therapies or treatment*)):ti,ab,kw and finally limited to publication dates 1990–2014.
For Prehabilitation questions, the following search strategies were used again in PubMed: Root Search: Neuroma, Acoustic [MeSH], (vestibular [Title/Abstract] OR vestibulocochlear [Title/Abstract] OR acoustic [Title/Abstract]) AND (neuroma* [Title/Abstract] OR neurilemmoma* [Title/Abstract] OR neurilemoma* [Title/Abstract] OR neurinoma* [Title/Abstract] OR tumor* [Title/Abstract] OR tumour* [Title/Abstract] OR schwannoma* [Title/Abstract]), #1 OR #2, Prehab* [TIAB] OR vestibular rehab* [TIAB] OR Preoperative Care [Mesh] OR Gentamicin [TIAB] OR Gentamicins [Mesh] OR Vestibular ablation [TIAB], (animal [MeSH] NOT human [MeSH]) OR cadaver [MeSH] OR cadaver* [Titl] OR comment [PT] OR letter [PT] OR editorial [PT] OR addresses [PT] OR news [PT] OR “newspaper article” [PT] OR case reports [PT] OR case report [Title], #5 AND English [Lang], and #6 AND (“1966/01/01” [PDAT] : “2015/01/01” [PDAT]). In addition, a Cochrane Central Search was performed using: MeSH descriptor: [Neuroma, acoustic] explode all trees, ((vestibular or vestibulocochlear or acoustic) and (neuroma* or neurilemmoma* or neurilemoma* or neurinoma* or tumor* or schwannoma*)):ti,ab,kw, #1 or #2, MeSH descriptor: [Preoperative care] explode all trees, MeSH descriptor: [Gentamicins] explode all trees, (Prehab* or vestibular rehab* or Gentamicin or Vestibular ablation):ti,ab,kw, and Publication dates: 1946-2014.
For searches, PubMed was used for surgical questions: Root Search: Neuroma, Acoustic [MeSH], (vestibular [Title/Abstract] OR vestibulocochlear [Title/Abstract] OR acoustic [Title/Abstract]) AND (neuroma* [Title/Abstract] OR neurilemmoma* [Title/Abstract] OR neurilemoma* [Title/Abstract] OR neurinoma* [Title/Abstract] OR tumor* [Title/Abstract] OR tumour* [Title/Abstract] OR schwannoma* [Title/Abstract]), #1 OR #2, Neurosurgical procedures [MeSH] OR Otologic surgical procedures [MeSH] OR Minimally invasive surgical procedures [MeSH] OR Microsurgery [MeSH] OR Surgery [SH] OR Resection [TIAB] OR microsurger* [TIAB] OR microsurgical [TIAB] OR surger*[tiab] OR surgical [tiab] OR operati* [tiab] OR suboccipital [TIAB] OR translabyrinthine [TIAB] OR middle fossa [TIAB] OR retrosigmoid [TIAB] OR transcochlear [TIAB] OR presigmoid [TIAB] OR transpetrosal [TIAB] OR extracisternal [TIAB] OR Treatment outcome [MH] OR outcome* [TIAB], Endoscopy [MH] OR Endoscop* [TIAB] OR Neuroendoscopy [MH] OR Neuroendoscopes [MH] OR neuroendoscop* [TIAB] , (animal [MeSH] NOT human [MeSH]) OR cadaver [MeSH] OR cadaver* [Titl] OR comment [PT] OR letter [PT] OR editorial [PT] OR addresses [PT] OR news [PT] OR “newspaper article” [PT] OR case reports [PT] OR case report [Title], English [Lang], and (“1946/01/01” [PDAT] : “2015/01/01” [PDAT]) In addition, a Cochrane Central Search was performed using: MeSH descriptor: [Neuroma, Acoustic] explode all trees, ((vestibular or vestibulocochlear or acoustic) and (neuroma* or neurilemmoma* or neurilemoma* or neurinoma* or tumor* or schwannoma*)):ti,ab,kw, #1 or #2, MeSH descriptor: [Neurosurgical procedures] explode all trees, MeSH descriptor [Otologic surgical procedures] explode all trees , MeSH descriptor [Minimally Invasive Surgical Procedures] explode all trees, MeSH descriptor [Microsurgery], MeSH descriptor [Treatment outcome] explode all trees, Any MeSH descriptor with qualifier(s): [Surgery - SU], (Resection or microsurger* or microsurgical or surger* or surgical or operati* or endoscop* or suboccipital or translabyrinthine or “middle fossa” or retrosigmoid or transcochlear or presigmoid or transpetrosal or extracisternal or outcome*):ti,ab,kw, MeSH descriptor: [Endoscopy] , MeSH descriptor: [Neuroendoscopy], MeSH descriptor: [Endoscopes], MeSH descriptor: [Neuroendoscopes], (Endoscop* or neuroendoscop*):ti,ab,kw, and Publication dates: 1946–2014.
Article Inclusion/Exclusion Criteria
Seventy-eight citations were manually reviewed by the team with specific inclusion and exclusion criteria as outlined below. Two independent reviewers reviewed and abstracted full-text data for each article, and the 2 sets of data were compared for agreement by a third party. Inconsistencies were rereviewed, and disagreements were resolved by consensus. Only citations that considered adult patients focusing on surgical treatment of VSs were considered. To be included in this guideline, an article has to be a report of a study that:
-
Investigated patients suspected of having VSs
-
Patients ≥18 years of age
-
Was in humans
-
Published between January 1, 1966 and December 31, 2014
-
Quantitatively presented results
-
Was not an in vitro study (for novel molecular markers, in vitro studies were included on patient samples)
-
Was not a biomechanical study
-
Was not performed on cadavers
-
Was published in English
-
Was not a meeting abstract, editorial, letter, or commentary
-
Studies may include mixed pathology; however, the data pertaining to VSs were abstractable from the paper.
-
5 patients or patient samples
The task force did not include systematic reviews, guidelines, or meta-analyses conducted by others. These documents are developed using different inclusion criteria than those specified in this guideline. Therefore, they may include studies that do not meet the inclusion criteria used for this guideline. The task force recalled these documents if their abstract suggested that they might address one of the recommendations, and the bibliographies were searched for additional studies.
Classification of Evidence and Guideline Formulation
The concept of linking evidence to recommendations has been further formalized by the American Medical Association (AMA) and many specialty societies, including the American Association of Neurological Surgeons (AANS), the Congress of Neurological Surgeons (CNS), and the American Academy of Neurology (AAN). This formalization involves the designation of specific relationships between the strength of evidence and the strength of recommendations to avoid ambiguity. In the paradigm for therapeutic maneuvers as used in this section, evidence is classified into that which is derived from the strongest clinical studies (eg, well-designed, randomized controlled trials), or class I evidence. Class I evidence is used to support recommendations of the strongest type, defined as level 1 recommendation, indicating a high degree of clinical certainty. Nonrandomized cohort studies, randomized controlled trials with design flaws, and case-control studies (comparative studies with less strength) are designated as class II evidence. These are used to support recommendations defined as level 2, reflecting a moderate degree of clinical certainty. Other sources of information, including observational studies, such as case series and expert opinion, as well as randomized controlled trials with flaws so serious that the conclusions of the study are truly in doubt are considered class III evidence and support level 3 recommendations, reflecting unclear clinical certainty. A basis for these guidelines can be viewed at: here.
Therapeutic
Classification of Evidence on Therapeutic Effectiveness and Levels of Recommendation
Evidence Classification
|
|
Class I
|
Evidence provided by one or more well-designed randomized controlled clinical trials, including overview (meta-analyses) of such trials
|
|
Class II
|
Evidence provided by well-designed observational studies with concurrent controls (eg, case-control and cohort studies)
|
|
Class III
|
Evidence provided by expert opinion, case series, case reports, and studies with historical controls
|
Levels of Recommendation
|
|
Level 1
|
Generally accepted principles for patient management, which reflect a high degree of clinical certainty (usually this requires class I evidence which directly addresses the clinical questions or overwhelming class II evidence when circumstances preclude randomized clinical trials)
|
|
Level 2
|
Recommendations for patient management which reflect clinical certainty (usually this requires class II evidence or a strong consensus of class III evidence)
|
|
Level 3
|
Other strategies for patient management for which the clinical utility is uncertain (inconclusive or conflicting evidence or opinion)
|
Results
Medical Therapy
Study Selection
For this section, 22 full-text articles were reviewed, and 7 were excluded (4 were animal studies, 1 as a repeat report of the same patients, and in 2, data pertinent to only VSs was not abstractable).
Bevacisumab (Avastin)
Question: What is the role of bevacizumab in the treatment of patients with VSs?
Study Characteristics and Results of Individual Studies
Three retrospective series of treatment-resistant VSs in the setting of NF2 are reported in patients treated with bevacizumab, which inhibits tumors as a vascular endothelial growth factor (VEGF)-binding antibody (Table 1). Alanin et al9 reported on 12 consecutive patients treated for a median treatment duration of 22 months and analyzed treatment response both radiographically and hearing response. In 6 of 12 patients (50%), a >20% decrease in tumor size was seen.9 This was maintained for >2 months in 33% of patients.9 Twenty-five percent of patients had objectively improved hearing.9 One patient died secondary to intracerebral hemorrhage secondary to treatment.9 Plotkin et al10,11 reported an initial series of 10 patients, then followed this up with a separate series of 31 patients. Objective hearing improvement was seen in 57% of patients.10,11 Radiographic response (>20% volume reduction) was seen in 55% of patients.10,11 The median time to response was 3 months. Ninety percent and 61% of patients had stable hearing at 1 and 3 years, respectively.10,11 Eighty-eight percent and 54% of patients had stable or smaller tumors at 1 and 3 years, respectively.10,11 There were no intracranial hemorrhages as a side effect of treatment.10,11 These studies represent small case series without randomization, and therefore are Class III evidence.
Risk of Bias Within Studies
Due to a lack of control arm in any study reporting the effects of bevacizumab, the fluctuation in tumor size and hearing is unknown in NF2 patients with treatment-resistant tumors. Therefore, it is also unknown whether this phenomenon occurs otherwise (although this is unlikely).
Synthesis of Results
Class III evidence suggests that bevacizumab results in >50% improved hearing and >50% objective radiographic shrinkage of the VSs in NF2 patients but is not effective in all patients.9–11 The duration of treatment appears to be prolonged, although there is eventual loss of effectiveness with continued treatment (Table 1).9–11
Additional Analysis/Future Research
Clearly, bevacizimab poses an intriguing option for therapy not only for treatment-resistant NF2, but by inference, may be an option for treatment-resistant sporadic VSs. However, larger studies and more experience needs to be accumulated, as well as better evidence. Ideally, a multi-institutional prospective randomized trial would be most definitive; however, due to the rarity of the disease, this may be impractical. The long-term toxicity is unknown, although the immediate risk of renal failure is potentially small. If this therapy is found to be effective, long-term treatment may pose unique side effects yet unknown. In addition, the cost of bevacizimab is an issue especially considering its long-term use, and thus will need to be carefully considered. Future research should also address the duration needed and if the tumors develop resistance to the drug as occurs with chemotherapies in other diseases. Moreover, the use of this drug with radiation would be another consideration to enhance radiation effectiveness. Currently there is a prospective trial registered with clinical trials.gov (NCT01767792) underway in the form of a phase 2 study of bevacizumab in NF2 with growing VSs, which started accrual on May 1, 2013.
Discussion
Treatment with bevacizumab was followed by a clinically relevant tumor-volume reduction and hearing improvement in some but not all patients treated with minimal treatment related side effects.9–11 Although there is a potential for life-threatening brain hemorrhage, this was seen in only one patient reported in these series to date.9 It is also useful to note that NF2 pathologic specimens stain for, and therefore, produce VEGF.11 Although the mechanism of action is unknown, it is presumed that bevacizumab inhibits VEGF-mediated angiogenesis within the tumor, resulting in its effect.
Summary of Evidence
All evidence available is class III.
Limitations (Question/Topic)
The question pertinent to treatment was easily searchable and limited, so while missed studies are possible, this is unlikely. Given the rarity of the disease, patients are quite limited, and small sample size may lead to some bias.
Conclusion
Based on the information at hand, recommendations that can be made include bevacizumab may be administered to radiographically reduce the size or prolong tumor stability in patients with NF2 without surgical options. Also, bevacizumab may be administered to improve hearing or prolong time to hearing loss in patients with NF2 without surgical options.
AR42
Question: What is the role of AR42, a histone deacetylase inhibitor, in the treatment of patients with VSs?
Study Characteristics
There is no clinical data regarding AR42, and because it was included in the search as an emerging therapy, it is presented here for completeness. Two studies are presently available involving first-pass VS specimens in mice using AR42.12,13 AR42, a novel histone deacetylase inhibitor, inhibits AKT downstream from PI3K.12,13 PI3K activation has been shown to be a consequence of the Merlin mutation in VSs.12,13 Treatment arms were designed in both studies to have a 1:1 treatment with AR42 to control treatment mice.
Risk of Bias Within Studies
These data represent human specimens treated within a murine model, therefore patient treatment is unknown.
Results of Individual Studies
Compared to control treatments, growth of first-pass tumor implants were significantly decreased by treatment with AR42. Tumor samples treated with AR42 demonstrated effective inhibition of AKT phosphorylation at treatment doses nontoxic to the mice.12,13 Moreover, treated samples demonstrated cell cycle arrest and apoptosis.12,13
Synthesis of Results
AR42 may inhibit VS growth by inhibition of AKT phosphorylation.
Risk of Bias Across Studies
Due to the controlled setting of a murine model, there is very little expected variance across studies.
Additional Analysis/Future Research
This preclinical research has initiated in a trial registered with clinical trials.gov (NCT02282917), which will be a phase 0 study of tumorcidal effect. Patients will receive treatment prior to resection. Prior to surgery, imaging will be performed to assess growth reduction, and postoperative pathology will assess its tumorcidal effect.
Discussion
Due to known intense activation of the PI3K pathway, this treatment is promising as a direct tumor inhibitor; however, currently there are no in-human data, and therefore no recommendations can be made.
Summary of Evidence
Preclinical research on primary in vivo first-pass tumor specimens of both VSs and meningiomas pertinent to NF2. AR42 may be effective in treating VSs as evident by these 2 murine studies treating primary patient tumor samples.12, 13
Imatinib Mesylate, Lapatinib, Erlotinib, Everolimus
Question: Is there a role for imatinib mesylate, lapatinib, erlotinib, or everolimus in the treatment of patients with VSs?
Study Characteristics
Imatinib mesylate (Gleevac): Preclinical study assessing imatinib mesylate, which inhibits platelet-derived growth factor receptor (PDGFR), was evaluated in 34 patient samples after resection.14 Samples were assessed for PDGFR expression and exposed to primary culture and treatment with imatinib mesylate.14
Lapatinib (Tykerb): Lapatinab is a small molecular weight inhibitor of EGFR and ErbB2 tyrosine receptor kinases.15,16 A preclinical study involving 11 patient samples screened after tumor resection for response to lapatinib is presented.15 Translating these findings, a clinical phase 2 single institutional prospective study of 21 NF2 patients with treatment-resistant tumors were treated with lapatinib was undertaken where hearing and tumor volumetric response were treatment endpoints.16
Erlotinib (Tarceva): Erlotinib functions as an EGFR receptor inhibitor. Eleven consecutive patients with treatment-resistant NF2 were treated with erlotinib.17
Everolimus (Zortess/Afinitor): Everolimus is an oral inhibitor of the mammalian target of rapamycin (mTOR) complex. Ten consecutive patients with treatment-resistant NF2 were treated with everolimus.18
Risk of Bias Within Studies
As there was no control arm in the erlotinib study, the fluctuation in tumor size and hearing is unknown in NF2 patients with treatment-resistant tumors. It is also uncertain whether this phenomenon occurs otherwise, although this is unlikely.
Results of Individual Studies
Imatinib mesylate (Gleevac): PDGFR expression was found in 23 (68%) of 34 samples.14 In primary culture, imatinib mesylate was found to downregulate activation of its corresponding tyrosine kinase pathway.14 Therefore, there may be a role for therapy.
Lapatinib (Tykerb): Preclinical testing of 11 patient samples treated with lapatinab demonstrated inhibition of phosphorylation of downstream ERK1/2 and AKT in 7(64%) of those 11 samples.15 In 21 patients treated prospectively, 24% had objective radiographic tumor shrinkage, and 31% had objective hearing improvement.16 Median time to tumor progression on treatment was 14 months supporting a prolonged treatment effect.16 Toxicity was minor (see Table 2 for summary).16 These data are class III data due to the small case series without randomization or comparison.
Erlotinib (Tarceva): No patient met objective criteria for tumor volume reduction or objective hearing improvement.17 Median time to tumor progression was 7.1 months and hearing worsening was 9.2 months.17 Toxicity was considered minor (see Table 3 for summary).17 These data are class III due to the small case series without randomization or comparison.
Everolimus (Zortess/Afinitor): No patient met objective criteria for tumor volume reduction/stabilization or objective hearing improvement.18 The study was terminated early due to inefficacy. (see Table 4 for summary).
Synthesis of Results
Imatinib mesylate (Gleevac): Imatinib mesylate may play a role in VS treatment, as PDGFR expression was found in 68% of patient samples.14
Lapatinib (Tykerb): Lapatinib demonstrated preclinical inhibition of its molecular-targeted pathway and further evidence of effective treatment resulting in objective hearing improvement in 31% of patients and volumetric reduction in tumor size in 24% of patients with a prolonged treatment effect.15,16
Erlotinib (Tarceva): Erlotinib had no treatment effect in this small series.
Everolimus (Zortess/Afinitor): Everolimus had no treatment effect in this small series, and the study was terminated early due to ineffectiveness.
Additional Analysis/Future Research
Imatinib mesylate (Gleevac): Preclinical research laid the groundwork for potential treatment of VSs. Logically, the next step would be treatment of treatment-resistant tumors, such as those we see in NF2.
Lapatinib (Tykerb): Further studies from more than one institution will be needed to confirm treatment effect. As with imatinib, it is unknown as to what the long-term toxicity of treatment would be. Since this is a small–molecular weight inhibitor, opportunity for combination therapy exists. Further, therapy in these studies was terminated after 12 cycles; perhaps treatment in responders can be extended longer.
Erlotinib (Tarceva)/everolimus (Zortess/Afinitor): Although there appears to be no treatment effect with erlotinib or everolimus, the patient series consisted of only 11 and 10 patients, respectively, providing room for reintroduction of therapy attempts in the future. Preclinical testing of treatment effects on tumor samples as has been shown with AR42, and imatinib mesylate may be of benefit prior to attempts at reintroducing this to treatment paradigms.
Discussion
Matinib Mesylate (Gleevac): PDGRF expression was not uniform in VSs; therefore, this treatment should rely on pretreatment post–resection analysis of the tumor itself for its expression. Then it may be a treatment option. Although there is no current patient treatment series, there are singular case reports available of its use in the current literature.
Lapatinab (Tykerb): Although the treatment effect seen in the prospective case series appears to be less than that of bevacizumab, in this early study it is hard to state that one is more effective than the other. More studies are needed to support one treatment having potential superiority over the other.
Erlotinib (Tarceva)/everolimus (Zortess/Afinitor): At this time, there appears to be no further role for human phase trials treating VSs with erlotinib or everolimus. While treatment numbers are small compared to other potential therapies, we would expect to see some hopeful treatment effect if this therapy is to be considered further.
Summary of Evidence
Class III: Lapatinib is effective in reducing VS size and improvement in hearing in patients with NF2
Class III: Erlotinib is ineffective in reducing VS size or improvement in hearing in NF2.
Class III: Everolimus is ineffective in reducing VS size or improvement in hearing in NF2.
Limitations
The limitations of these studies are that they represent preclinical and early clinical studies. Due to the rarity of VSs, it will be hard to compile large randomized studies, but these studies will have to be expanded to multi-institutional studies to show continued efficacy and generalizability in those agents that have shown effectiveness.
Conclusion
Molecular pathway specific treatment of VSs appears likely in the near future. Multiple agents as demonstrated above are in various stages of preclinical and clinical trials of feasibility and effectiveness. Imitinib mesylate has been shown in preclinical work to have the potential to treat VSs.14 Lapatinib in a human trial demonstrated effectiveness in tumor volume reduction and objective hearing improvement.15,16 Erlotinib and everolimus appear to be ineffective in reducing VS size or improvement of hearing in a small series.17, 18
Aspirin
Question: What is the role of aspirin, to augment inflammatory response, in the treatment of patients with VSs?
Study Characteristics
Aspirin is a cyclo-oxygenase inhibitor, of which recent evidence suggests tumor growth may be driven by inflammatory processes.19 Guided by evidence that VSs possess within them a considerable amount of inflammatory cells, especially macrophages, inflammatory inhibition via aspirin has been investigated as a potential treatment for VSs undergoing observation.19 Kandathil et al19 reported a retrospective case-control series of 687 tumors, of which 347 were observed. Only 81 patients took aspirin (summary in Table 5).19
Risk of Bias Within Studies
The risk of bias within this study is very high, because it was retrospective, and patients were not randomized to aspirin; therefore, the difference between treatment groups was not accounted for. Although the observation between the groups is striking, some bias could have been controlled for by matched pair analysis however this still likely given what appears to be a higher proportion of comorbidities in these patients. It should be noted that those undergoing observation were older at baseline, 69 years compared to 63 years.19 In controlling for age, the authors still found a difference. Tumor size was similar between groups.19
Synthesis of Results
Of 266 nonaspirin users, 154 (58%) demonstrated growth.19 Of the 81 aspirin users, 33 (41%) demonstrated growth (Table 5).19 Aspirin is associated with less risk of tumor progression, with an odds ratio of 0.5 and a confidence interval of 0.29-0.85.19 Therefore, aspirin use may be useful in patients with VSs undergoing observation; however, this study should be validated in prospective randomized trials that should be achievable.
Additional Analysis/Future Research
Again, given the retrospective nature of the Kandathil et al19 study and the relative understanding and ubiquity of aspirin treatment, this study needs to be validated with at least a prospective trial of patients being randomized at the time of observation for their VS. Ideally, to minimize confounders, this study should have been multi-institutional and should be achievable. In addition, in patients undergoing a subtotal resection and electing observation, there may have been additive value to adding aspirin as another potential treatment option. A better understanding of the mechanism of action for aspirin treatment and growth inhibition would lend itself to in vivo animal testing as well.
Discussion
Kandathil et al19 provided an intriguing article in that they recognize that frequently VSs are found cohabitating with macrophages.20 Given this association with inflammatory cells, they postulated that a form of inflammatory inhibition thru the cyclo-oxygenase pathway by treatment with aspirin may result in reduced tumor growth.19 In patients treated with aspirin, only 41% demonstrated growth that was evident on MRI, while 58% of those not receiving aspirin demonstrated growth. This difference represents quite a statistically significant argument; however, this study remains class III evidence.
Summary of Evidence
Class III: Available retrospective evidence suggests that aspirin use in patients undergoing observation may reduce future risk of growth based on interference of inflammatory cascade.
Limitations
As stated in potential biases, this study by its nature is a retrospective study and shows a statistically significant, albeit small, effect. Ideally, continued research on this topic will eliminate limitations on generalizability and control interpatient differences in a prospective manner.
Conclusion
Aspirin therapy in patients undergoing observation may reduce the risk of growth of the index VSs; however, further validation is required.
Vasospasm
Question: Is there a role for treatment of vasospasm (ie, nimodipine or hydroxyethyl starch) perioperatively to improve facial nerve outcomes in patients with VSs?
Study Characteristics
A series of studies written by the same primary or corresponding author, Dr Scheller, are presented. In their first study, of 45 patients undergoing surgery, 25 underwent therapy with both nimodipine and hydroxyethyl vasoactive treatments starting at the time of surgery and lasting 10 days postoperatively.21 Mean age and tumor size were not statistically different.21 In a separate study of new consecutive patients between 2004 and 2006, 30 patients were enrolled prospectively, and all received vasoactive treatment with nimodipine and hydroxyethyl; however, 14 received therapy a day prior to surgery and 16 received therapy initiated either during the surgery (9) or did not receive therapy (7).22 Preoperative tumor characteristics and demographics were similar, and the effect on facial and cochlear nerve was assessed.22 Continuing their research in what appears to be a separate series between 2007 and 2009, 37 patients were treated with prophylactic preoperative nimodipine only; however, 20 were given intravenous (IV) doses nimodipine necessitating a preoperative admission, and 17 were given oral nimodipine for 10 days to assess which is more efficacious.23
Risk of Bias Within Studies
Due to the variability among presenting VSs, the authors concluded that although tumor size and age may be similar among groups in a small series, tumor consistency, vascularity, and the course of the seventh and eighth nerves are so variable that with these small case numbers, it is difficult to truly distill the effects of vasoactive treatment. Therefore, given that this particular author has produced many reports regarding its use and that the practice has not proliferated outside of the author’s institution, there may be intrinsic bias within the report.
Results of Individual Studies
Strauss et al21 report in the first study of this series that in 45 patients, of the 25 receiving treatment 15 had a postoperative House–Brackmann score ≤5, and without treatment, 8 had a similar result with only 3 receiving less than complete resections.21 At 1 year, only 2 patients (13%) did not recover facial function in the vasoactive treatment group, while 5 (63%) did not recover facial function in the nonvasoactive treatment arm. This difference produced a P value of 0.002.21 The authors noted no difference in hearing preservation with treatment.21 In a subsequent study by Scheller et al22 that compared preoperative to intraoperative or no vasoactive treatment, in those patients with prophylactic vasoactive treatment, 58% had preserved hearing and no long-term facial weakness worse than a House–Brackmann score of 2, while in patients without prophylactic treatment, only 15% had preserved hearing (P = .041) and 38% had long-term facial weakness (P = .045).22 As a follow-up to this study, patients were treated with varying oral compared to IV nimodipine, here it was found that cerebrospinal fluid (CSF) levels of nimodipine were higher with IV and that facial nerve outcomes were better with IV treatment (P = .038), although there were no differences in hearing preservation.23 This information is summarized in Table 6.
Synthesis of Results
Through these small series, there appears to be a consistent positive effect of treatment with vasoactive agents, specifically nimodipine, on the outcome of the facial nerve over the long term and potentially on hearing preservation. Although IV therapy is likely more efficacious, oral therapy may be of use as well and would mitigate the cost of IV therapy for 1 day preoperatively and 10 days postoperatively. While these results are encouraging, studies outside the authors’ institution are needed to validate results.
Risk of Bias Across Studies
There is substantial worry for bias among these reported studies.21–24 Given that all of the series have small numbers, rather than expanding on a working protocol of vasoactive treatment, a series of smaller studies with many variables are presented, which makes it difficult to draw sound conclusions regarding vasoactive treatment and rarely controlling for the variability of the tumor itself.
Additional Analysis
These studies lay the groundwork for considering protocols of nimodipine or other vasoactive treatments perioperatively in VS surgery; however, there is much to be done to make this standard. A larger study that is well controlled would be a next step towards a multi-institutional randomized study, which would be achievable given the large number of VS resections ongoing every year.
Discussion
It is interesting to note that vasoactive treatment may improve postoperative cranial nerve outcomes, theoretically by minimizing postoperative ischemia. In the treatment of subarachnoid hemorrhage, nimodipine is well known not to affect the likelihood of vasospasm; however, the authors believe it has a protective effect on brain function, which may also be the case here. Intravenous administration may be prohibitive in today’s cost environment. Therefore, in order to achieve the results as presented in these papers, oral therapy (albeit potentially less effective) may be more attractive.21–24 If the addition of such a low toxicity treatment does in fact have such a significant treatment effect that these small studies demonstrate it repeatedly, then strongly treating patients with vasoactive treatments more routinely perioperatively should be considered. Again, there appears enough evidence here to push for well-designed trials around perioperative vasoactive treatments.
Summary of Evidence
Class III: Perioperative treatment with nimodipine (or with addition of hydroxyethyl starch) appears to improve postoperative facial nerve outcomes and may improve hearing outcomes.
Conclusion
Further research is needed to ascertain the true effect of vasoactive treatments for perioperative improvement in long-term facial nerve and cochlear nerve outcomes; however, treatment may be considered to attempt to achieve this effect in one’s individual practice currently. Therefore, we recommend enrollment in properly designed clinical trials to address this question.
Prehabilitation: Preoperative Vestibular Rehab
Question: Is there a role for preoperative vestibular rehab or vestibular ablation with gentamicin for patients surgically treated for VSs?
Study Selection
Four papers were identified by the literature search, 2 of which were usable for analysis because they provide data relative to VS patients evaluated in a prospective manner.
Study Characteristics
Two cases series by Magnusson and colleagues evaluated the possibility of preoperative vestibular rehabilitation (euphemistically called prehab) and gentamicin ablation of the vestibular apparatus preoperatively. The first study by Tjemstrom et al25 details 41 patients undergoing translabyrinthian VS surgery. Group 1 (n = 17) had no preoperative vestibular function. Group 2 (n = 8) had vestibular function; however, gentamicin was not used to ablate the vestibular system. Group 3 (n = 10) had central vestibular disturbance and was not treated with gentamicin Group 4 (n = 6) had preoperative vestibular function, and gentamicin was administered.25 In the second study by Magnusson et al,26 the authors demonstrated the feasibility of preoperative gentamicin ablation of all 12 patients in this case series without analysis of length of hospital stay.
Risk of Bias Within Studies
There appears to be some selection bias as intertreatment baseline characteristics were not described.
Results of Individual Studies
Posturography following surgery at 6 months demonstrated less sway for group 4 patients, or those undergoing prehab and gentamicin ablation, which was statistically better than comparison groups.25 There was no analysis of hospital length of stay, which may be most significantly affected.25 In the second study by Magnusson et al26 there is noted improvement in “patients up and walking on their own on the first postoperative day.” However, no length of stay data were reported.26 It should be noted that patients took time off preoperatively after gentamicin injection.26 This information is described in additional detail in Table 7.
Synthesis of Results
These data provide preliminary results that prehab and preoperative gentamicin ablation of the vestibular apparatus may improve postoperative recovery; however formal, data regarding recovery are lacking.
Additional Analysis
While these treatments are intriguing, significant questions remain, such as the effectiveness of gentamicin ablation in providing a complete vestibular ablation. Further length of stay and a reduction in complications for the postoperative vestibulopathy need to be investigated. Prehabilitation offers a unique opportunity, but needs to be defined better. Fortunately, there are 3 ongoing registered patient trials (Table 10) on clinical trials.gov, the first being NCT02379754, which is studying gentamicin prior to schwannoma surgery in patients with residual vestibular function. A second trial, NCT02415257, is studying gentamicin prior to schwannoma surgery in those with no residual vestibular function. And finally, NCT02275325 investigates preoperative vestibular rehabilitation effectiveness after VS surgery.27
Discussion
VS resection in patients with significant vestibular function is often complicated postoperatively by significant vestibulopathy often preventing early mobilization leading to potential complications such as DVT or constipation. Further postoperative vestibulopathy may prolong recovery. The concept of preoperatively training patients to compensate for the postoperative vestibulopathy through prehab is intriguing. Moreover, ablation of the vestibular apparatus with gentamicin potentially offers a unique solution to difficulty in postoperative mobilization. These data provide information that these treatments may be useful in improving vestibular function postoperatively.
Summary of Evidence
Class III: Vestibular rehabilitation preoperatively aids in postoperative mobility.
Class III: Preoperative gentamicin ablation of the vestibular apparatus improves postoperative mobility.
Limitations
The small samples sizes of 6 and 12 patients restrict extrapolation of these results to larger series beyond that of feasibility at this time. In addition, patients may not be able to miss work preoperatively to undergo ablation of the vestibular apparatus, because this may fragment their recovery.
Conclusion
Prehab and preoperative gentamicin ablation may provide unique opportunities in improving postoperative mobility for patients undergoing VS surgery where they have preoperative vestibular function.
Surgical Therapy: Endoscopy
Question: Does endoscopic assistance make a difference in resection or outcomes in patients with VSs?
Target population: VS patients who are surgical candidates. Inclusion in this analysis required resection using the endoscope, either as the primary operative visualization or microscopic assistance.
Study Selection and Characteristics
Seventeen papers were identified for text screening, of these 8 met inclusion criteria and are presented. All studies were case series from a single surgeon; however, this author was representing separate institutional experiences. There were substantial differences in how the endoscopic technique was applied across groups, with the larger portion using the endoscope for the whole resection28,29; however, smaller series used the endoscope to assess the fundal portion of the tumor after retrosigmoid with internal auditory canal drillout to assess for further tumor or for air cells. One study by Chovanec et al30 was a prospective case-control; however, it was not randomized and was based on patient selection of the use of the endoscope prior to the operation.
Risk of Bias Within Studies
Given that these studies are individual patient series presenting a new technique, there may be some conflict in the reporting of results for self-promotion; however, the peer review process and academic integrity should minimize this possibility. Moreover, new results typically tend to emphasize short-term results, and the longevity of the results are unknown. However, given the concept of gross total resection compared to less than gross total resection is not unique to endoscopic resection, this would mitigate this difference.
Results of Individual Studies
The largest study is Dr Shahinian’s study, which published his initial results in 112 patients and then continued the results in his series published in 2011 with 527 tumors.28,29 This retrospective case series presented tumor resection wholly with endoscopic resection with a mean size of 2.8 cm (range 0.3–5.8 cm) and with a mean follow-up of 49 months.28,29 Ninety-four percent of patients were believed to have a gross total resection with a relatively high recurrence rate of 7.4% (although with high hearing preservation rates which have a higher rate of recurrence) at such a short follow-up period.28,29 Ninety-five percent of patients had House–Brackmann scores ≥2 at 1 year, and 57% of patients had useful hearing preservation with no deaths or cases of meningitis.28,29 There was a relatively low rate of CSF leaks at 3.2% along with a short operative time reported at 132 minutes.28,29 In an interesting trial design, Chovanec et al30 discussed the risks and benefits of both microscopic and endoscopic surgery with their patients and allowed them to choose their preference in a prospective manner in 89 patients from 2008 to 2010. Forty-four patients selected microsurgery (mean size 2.8 cm, mean age 45 years) and 39 received endoscope assistance (mean size 2.6 cm, mean age 47 years) with 6 patients dropping out. Those selecting endoscopic surgery had better facial nerve outcomes (80% vs 58%), better useful hearing preservation (21% vs 2%), and lower rates of CSF leaks (8% vs 20%).30 The gross total resection rates were the same at 100% with the only recurrence occurring in the microsurgery group (2%) at a mean follow-up of 28 months.30 Goksu et al31 reported their experience from 1996 to 2002 in 60 patients undergoing endoscope-assisted resection with a mean tumor size of 2.3 cm (range 0.6–5 cm). They report 63% good facial outcome and 24% hearing preservation with no recurrences; however, the follow-up interval was not reported.31 There were 8 CSF leaks (13%), which appears to be a higher rate of CSF leaks than would be expected.31 Magnan et al32 report 119 patients treated from 1993 to 1998 with all tumors being <2.5 cm. Good facial function was seen postoperatively in 96% of patients. Thirty percent experienced useful hearing preservation, and the CSF leak rate was 12% with no deaths.32 Gerganov et al33 reported an interesting case series looking at the intraoperative changes in electrophysiology postulating that heating from the endoscope may impact outcome by comparing 30 consecutive endoscopic-assisted cases to historical controls. Here the authors concluded that with proper use and precaution the introduction of the endoscope does not increase intraoperative complications nor affect electrophysiology. Additional information on these studies is presented in Table 8.
Synthesis of Results
It is clear that introducing the endoscope to the procedure as either the primary visualization or as supplemental visualization appears safe. However, the true advantages of the technique are unknown and may improve operative outcomes for facial nerve preservation and useful hearing preservation. In addition, the authors claim an improvement in CSF leak incidence with the technique; however, some series report CSF leak rates similar to microscopic resection; therefore, although this may be a benefit, there is insufficient evidence to recommend endoscopic resection to avoid CSF leaks.
Risk of Bias Across Studies
There is substantial risk of bias across studies as the technical skill of the surgeon is expected to be different, and the experience of the surgeon will play a role. In addition, as technologies are introduced, they get better with time. The equipment used in earlier studies is clearly not the same as the more sophisticated equipment of the mid- to late 2000s that was introduced with the expansion of endoscopic skull base surgery.
Additional Analysis
Understanding the role of endoscopic assistance in VS surgery will likely be a moving target and evolve with time, similar to the evolution of endovascular techniques for aneurysms. One impending issue with comparing these techniques is advancing technologies in both fields. Endoscopes are clearly evolving, and newer cranial-assisted endoscopes are arriving yearly. Also, the introduction of cold light source would generally advance this technology. In addition, microscopes are undergoing changes at this time, and operative fluorescence is not a technique that has yet arrived to VS surgery. However, if a fluorescence was developed that could stain the course of the facial or cochlear nerve, additional research in endoscopic technique would be inconsequential. To understand if there is a true difference in technique, one would have to perform a multi-institutional study comparing techniques among surgeons.
Discussion
A meta-analysis comparing primary outcome of facial nerve preservation with secondary outcome of hearing preservation, gross total resection, CSF leak, wound infection, recurrence, and death was reported by Alobaid et al,34 who compiled these results. This analysis encompassed all papers published from 1996 to 2011 that were performed through a retrosigmoid craniotomy. There were 790 endoscopic cases (although they failed to recognize 112 of 527 in Dr Shahinian’s overlapping case series28,29) compared to 3026 microsurgical cases reported in the same time period.34 In the endoscopic group, the mean tumor size was 2.7 cm, and the gross total resection rate was 97% compared to 2.5 cm and 91% in the microscopic group respectively.34 Good facial nerve outcomes (House–Brackmann ≥2) were present in 94% of endoscopic cases and in 67% of microsurgical cases. Useful hearing preservation using a >50% word recognition was seen in 46% and 23% of endoscopic cases compared to microsurgical cases, respectively.34 Recurrence was higher in the microscopic group at 2.6% compared to 1.3% in the endoscopic group, but the follow-up duration was unclear.34 This report is to be taken with a grain of salt because 81% of the cases presented in the endoscopic group were from 1 surgeon.34 Among these studies, Chovanec et al30 stands out as a single surgeon using a new technique who is able to overcome the technical challenges of operating under an endoscope rather than a microscope and still have improved outcomes.30
Summary of Evidence
Although endoscopic assistance and pure endoscopic surgery for VS are recognized as emerging surgical techniques, they are not therapy and the surgeon is free to choose the type of surgical therapy they would prefer. The summary of evidence here suggests that endoscopic use for VS surgery does not appear to worsen outcome or complications. In addition, endoscopic assistance may aid in the prevention of postoperative CSF leaks by directly visualizing them for repair.
Limitations
Endoscopic tumor resection is a skill set that is not necessarily learned quickly, nor are microscopic skills easily transferable to the technique; therefore, it may be difficult for surgeons in general to adopt the technique unless they use the skill in other areas of their practice. In addition, both comparison arms of these studies are technology dependent, and one would expect further developments with both devices.
Conclusion
Endoscopic primary resection or assistance may aid in visualization of additional tumor in the fundus, as well as aid in better postoperative facial nerve and cochlear nerve outcomes; however, evidence is relatively weak to recommend its use in standard microscopic resection. There may be a reduction in CSF leaks with endoscopic evaluation of the petrous bone prior to closure.
Pathology: Molecular/Histological Markers
Question: Are there molecular or histologic markers that can predict response to or guide targeted medical therapies of VSs?
Study Selection
Thirty-five papers were screened for this section, and 1 paper met inclusion criteria; however, 5 overlapped with medical therapies and are included in that section above.
Study Characteristics
One study was identified as a multi-institutional study that retrospectively evaluated 49 schwannomas (36 sporadic and 13 with NF2) compared with 7 normal control vestibular nerves.35 The tumors were subjected to genetic and expression analysis along with microarray and clustering analysis.35
Risk of Bias Within Studies
This is a well-controlled patient sample analysis of gene and protein expression that may be susceptible to selection bias in that the tumors analyzed presumptively were large enough to spare tumor for research. However, the samples themselves were rigorously analyzed with a good control.
Results of Individual Studies
Upregulation of the PI3K and mTOR pathways was found to be significant (P < .001) as well as RAC, CDC42, and amyloid processing (P < .05).35 Micro-RNA processing, reactive oxygen species scavenging, and MYCN signaling were significantly downregulated.35 There appeared to be no difference in expression between sporadic and NF2-associated tumors.35 Additional information is provided in evidence Table 9.
Synthesis of Results
Several pathways of pathologic activation were found relative to normal vestibular nerve, such as PI3K and mTOR. Therefore, directed therapy regarding these pathways may offer medical therapy for treatment-resistant tumors.
Additional Analysis
This detailed analysis provides information to guide potential therapeutic studies. The numbers could be expanded but appear adequate for this type of analysis. Direct gene sequencing may offer more understanding of the specific pathologic forms of pathway activation.
Discussion
These types of analyses provide further rationale for in-patient testing and are critical for an understanding of tumor growth and occurrence. As in our analysis of everolimus above, an inhibitor of mTOR, 10 patients were treated without evidence of effectiveness; however, this does not close the door on its use as a combination therapy. PI3K is being further analyzed in a current ongoing trial with AR42 providing rationale for this treatment; however, there are other small–molecular weight inhibitors within this pathway that may prove useful.
Summary of Evidence
No human evidence: In human samples, PI3K and mTOR may be potential targets for treatment, but there is no evidence to support a clinical recommendation.
Limitations
This analysis assesses postresection tumors and does not imply clinical effectiveness. Therefore, the presence of a pathologic pathway capable of inhibition is encouraging. Additional studies are needed.
Conclusion
Pathologically abnormal pathways are present within VS samples compared to normal vestibular nerves, which may offer hope for single agent or combination direct pathway inhibition in treatment-resistant tumors.
Discussion
The clinical and preclinical work done thus far in regard to emerging therapies in VS treatment has not provided data that immediately translate into Level 1 recommendations at this time. Some progress has been made in medical therapies for treatment-resistant VSs and applies most usefully to patients with NF2 where preservation of function is key. Bevacizumab has made the most progress and appears to be a viable treatment option for patients with NF2 and growing tumors, or loss of hearing. In these patients, bevacizumab recovers some useful hearing function and results in tumor reduction; however, the effect is ultimately lost with time succumbing to the natural tendency of the tumor to grow, opening doors for combination or other therapies. Lapatinab also appears to have some effectiveness in tumor size reduction and hearing preservation.
Conclusions and Key Issues for Future Investigation
Other direct molecular inhibitors are also being investigated, and it appears that PI3K and mTOR pathway inhibitors hold the most promise. Further options for treatment include vasoactive treatments perioperatively to improve postoperative outcome and aspirin therapy in those patients undergoing observation in preventing growth. What is clear when considering medications being useful for VSs is that much more work is needed, and tumor consortiums should focus their efforts on promoting multi-institutional studies investigating these therapies. Furthermore, there are ongoing trials formally evaluating prehab and preoperative gentamicin ablation. Hopefully these will define the role of these therapies in improving postoperative balance functions and aiding patients’ postoperative recovery. In terms of endoscopic surgery for VSs or its use as an adjunct at the time of surgery, the jury is still out; however, for those comfortable with these techniques, there may be an advantage in improving facial and cochlear nerve outcomes, as well as reducing CSF leaks. It is important to note as a community of surgeons treating these conditions, it is critical to promote well-designed clinical trials (a list of ongoing trials is presented below in Table 10) to answer these questions and improve the outcomes of patients.
Conflict of Interest (COI)
The Vestibular Schwannoma Guidelines Task Force members were required to report all possible COIs prior to beginning work on the guideline, using the COI disclosure form of the AANS/CNS Joint Guidelines Committee, including potential COIs that are unrelated to the topic of the guideline. The CNS Guidelines Committee and Guideline Task Force Chair reviewed the disclosures and either approved or disapproved the nomination. The CNS Guidelines Committee and Guideline Task Force Chair are given latitude to approve nominations of Task Force members with possible conflicts and address this by restricting the writing and reviewing privileges of that person to topics unrelated to the possible COIs. The conflict of interest findings are provided in detail in the companion introduction and methods manuscript (here).
Disclaimer of Liability
This clinical systematic review and evidence-based guideline was developed by a multidisciplinary physician volunteer task force and serves as an educational tool designed to provide an accurate review of the subject matter covered. These guidelines are disseminated with the understanding that the recommendations by the authors and consultants who have collaborated in their development are not meant to replace the individualized care and treatment advice from a patient's physician(s). If medical advice or assistance is required, the services of a competent physician should be sought. The proposals contained in these guidelines may not be suitable for use in all circumstances. The choice to implement any particular recommendation contained in these guidelines must be made by a managing physician in light of the situation in each particular patient and on the basis of existing resources.
Disclosures
These evidence-based clinical practice guidelines were funded exclusively by the Congress of Neurological Surgeons and the Tumor Section of the Congress of Neurological Surgeons and the American Association of Neurological Surgeons, which received no funding from outside commercial sources to support the development of this document.
Acknowledgments
The authors acknowledge the Congress of Neurological Surgeons Guidelines Committee for its contributions throughout the development of the guideline and the American Association of Neurological Surgeons/Congress of Neurological Surgeons Joint Guidelines Committee for its review, comments, and suggestions throughout peer review, as well as Trish Rehring, MPH, CHES, and Mary Bodach, MLIS, for their assistance. Throughout the review process, the reviewers and authors were blinded from one another. At this time, the guidelines task force would like to acknowledge the following individual peer reviewers for their contributions: Sepideh Amin-Hanjani, MD, D. Ryan Ormond, MD, Andrew P. Carlson, MD, Kimon Bekelis, MD, Stacey Quintero Wolfe, MD, Chad W. Washington, MD, Cheerag Dipakkumar Upadhyaya, MD, and Mateo Ziu, MD.
Figures
Figure 1. Article flowchart.
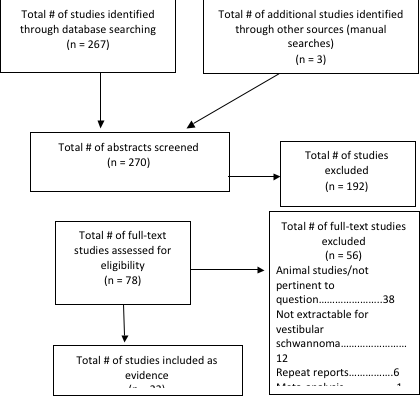
Table 1.Bevacizumab (Avastin)
|
Author (Year)
|
Study Description
|
Data Class
|
Conclusion
|
|
Alanin et al9 (2014)
|
12 consecutive patients with NF2 were treated with 10 mg/kg bevacizumab (a VEGF- binding antibody) every other week for 6 months. Growth and audiometry were followed.
|
III
|
The median treatment duration for the 12 patients was 22 months. In 6 of 12 patients (50%), a >20% decrease in tumor size was seen. This was maintained for more than 2 months in 33% of patients. 25% had objectively improved hearing. One patient died secondary to intracerebral hemorrhage secondary to treatment.
|
|
Plotkin et al11 (2009)
|
10 patients were treated in this initial series, all with treatment-resistant NF2. Response criteria were hearing improvement of >10 dB and reduction in tumor size of >20% of pretreatment tumor volume.
|
III
|
>50% of patients improved hearing with treatment with a sustained response on treatment. 90% of tumors shrank, which maintained in 40% of patients to 11 months. Therefore, bevacizumab appeared to have efficacy in treatment for this difficult patient population.
|
|
Plotkin et al10 (2012)
|
Bevacizumab treatment in 31 consecutive NF2 patients is reported retrospectively. Hearing improvement and tumor shrinkage were the primary endpoints.
|
III
|
Objective hearing improvement was seen in 57% of patients. Radiographic response (>20% volume reduction) was seen in 55% of patients. Median time to response was 3 months. 90% and 61% of patients had stable hearing at 1 and 3 years respectively. 88% and 54% of patients had stable or smaller tumors at 1 and 3 years respectively. There were no intracranial hemorrhages as a side effect of treatment.
|
NF2, neurofibromatosis type 2; VEGF, vascular endothelial growth factor.
Table 2.Lapatinib (Tykerb)
|
Author (Year)
|
Study Description
|
Data Class
|
Conclusion
|
|
Karajannis et al16 (2012)
|
Single institutional prospective phase 2 study of this EGFR and ErbB2 receptor tyrosine kinase inhibitor. 21 patients with NF2 were enrolled. Hearing and radiographic tumor response were treatment endpoints.
|
III
|
24% of patients had tumor shrinkage. 31% had objective hearing improvements. Median time to overall progression was 14 months supporting an inhibitory effect. Toxicity was considered minor.
|
NF2, neurofibromatosis type 2; VEGF, vascular endothelial growth factor.
Table 3.Erlotinib (Tarceva)
|
Author (Year)
|
Study Description
|
Data Class
|
Conclusion
|
|
Plotkin et al17 (2010)
|
Erlotinib is an EGFR receptor inhibitor. 11 consecutive patients with treatment-resistant NF2.
|
III
|
No patient met objective criteria for tumor shrinkage or hearing improvement. Median time to progression was 7.1 months and hearing worsening was 9.2 months. Erlotinib was felt to have no treatment effect in this small series.
|
NF2, neurofibromatosis type 2; VEGF, vascular endothelial growth factor.
Table 4. Everolimus (Zortress/Afinitor)
|
Author (Year)
|
Study Description
|
Data Class
|
Conclusion
|
|
Karajannis et al18 (2014)
|
Everolimus is an oral inhibitor of the mammalian target of rapamycin complex. This prospective study recruited patients for continuous treatment over 28 days.
|
III
|
10 patients were recruited. No patient responded to therapy with either an improvement or stabilization in hearing nor radiographic stabilization of their tumor size. The study was terminated early because of inefficacy.
|
Table 5. Aspirin
|
Author (Year)
|
Study Description
|
Data Class
|
Conclusion
|
|
Kandathil et al19 (2014)
|
Retrospective case series,
1980 to 2012. Of 687 tumors, 347 were observed. 81 of these patients took aspirin regularly.
|
III
|
Of the 266 nonaspirin users, 154 (58%) demonstrated growth. Of the 81 aspirin users, 33 (41%) demonstrated growth. This resulted in an odds ratio of 0.5, and confidence interval of 0.29-0.85 in favor of aspirin treatment during observation. The authors conclude that aspirin may play a role in preventing growth of spontaneous vestibular schwannomas undergoing observation.
|
Table 6. Vasospasm treatment
|
Author (Year)
|
Study Description
|
Data Class
|
Conclusion
|
|
Scheller et al23 (2014)
|
A comparative retrospective cohort study of 37 patients operated for VS resection. 17 received PO nimodipine and 20 received IV nimodipine on the day of surgery and 7 days postoperatively.
|
III
|
Significantly better facial nerve outcome in IV nimodipine group (P = .038). No difference for hearing outcome was found.
|
|
Scheller et al36 (2014)
|
Retrospective cohort study of 57 patients operated for skull base lesions. 25 received PO nimodipine and 32 received IV nimodipine.
|
III
|
Serum, CSF, and tissue levels of nimodipine were significantly higher after IV administration of nimodipine than after PO.
|
|
Scheller et al22 (2007)
|
Prospective open label randomized study comparing pre- and postoperative treatment with nimodipine and hydroxyethylstarch (n = 14) versus treatment at surgery (9) or no therapy (7) (n = 16) in patients undergoing VS surgery.
|
II
|
Prophylactic treatment with nimodipine and hydroxyethylstarch shows significantly better results for facial and cochlear nerve preservation during VS surgery (P = .045 and P = .041, respectively). Prophylactic treatment is superior to intraoperative; however, this study is underpowered.
|
|
Strauss et al21 (2006)
|
Retrospective cohort study of 45 patients undergoing VS surgery. 25 patients received nimodipine and hydroxyethyl starch, and 20 did not.
|
III
|
Long-term facial nerve outcome was significantly improved in those patients who experienced severe postoperative deterioration of facial nerve function and received vasoactive treatment compared to those who did not receive vasoactive treatment (13% vs 63%, P = .002). There was no difference in hearing preservation between groups.
|
CSF, cerebrospinal fluid; IV, intravenous; PO, per os; VS, vestibular schwannoma.
Table 7.Prehabilitation and preoperative gentamicin ablation
|
Author (Year)
|
Study Description
|
Data Class
|
Conclusion
|
|
Tjernstrom et al25 (2009)
|
Retrospective case series, 2001–2007. 41 patients undergoing translabyrinthine AN surgery. Group 1 (n = 17) with no preoperative vestibular function. Group 2 (n = 8) with vestibular function and no gentamicin given. Group 3 (n = 10) with central disorder. Group 4 (n = 6) with preoperative vestibular function and gentamicin given. Mean size not given, but within groups, those with remaining vestibular function were smaller as expected.
|
III
|
Posturography after surgery (6 months postoperatively) was best for group 4 compared to all other groups suggesting that separating the timing of surgery and vestibular differentiation improves long-term balance function. Authors do not mention length of hospital stay or length of time off following surgery. Intriguing idea that intuitively should work. Will need more data to make definitive conclusion.
|
|
Magnusson et al37 (2011)
|
Retrospective case series, years not described. 12 patients with preoperative vestibular function who underwent vestibular rehab and gentamicin injection before surgery.
Tumor size was not given.
|
III
|
“Patients were up and walking on their own on the first postoperative day.” No data on length of hospital stay. Patients took time off preoperatively after gentamicin injection. (Most were back at work within 2 weeks after treatment.) Intriguing idea that intuitively should work. Will need more data to make definitive conclusion.
|
AN, acoustic neuroma.
Table 8. Endoscopic visualization in vestibular schwannoma resection
|
Author (Year)
|
Study Description
|
Data Class
|
Conclusion
|
|
Kabil et al28 (2006)
|
Retrospective case series, 2001–2005. 112 tumors in 112 patients all retrosigmoid with only EA. Mean size = 2.6 cm (range 0.6–5.7 cm).
|
N/A
|
58% of patients with useful preoperative hearing (59/101)
95% good facial outcome at 1 year (HB 1 or 2)
Resection: GTR = 95%, STR = 5%
Recurrence rate = <1%
Mean follow-up: 17 months
Complications (n): 0 deaths, 3 CSF leaks, 0 meningitis cases, 3 superficial wound infections
The authors conclude that the endoscope provides improved visualization, smaller craniotomies, decreased operative time (132 minutes), and decreased complications.
|
|
Shahinian et al29 (2011)
|
Retrospective case series, 2001–2010.
527 tumors in 527 patients all retrosigmoid with only EA. Mean size = 2.8 cm (range 0.3–5.8 cm)
|
N/A
|
57% of patients with useful preoperative hearing (59/101)
95% good facial outcome at 1 year (HB 1 or 2)
Resection: GTR = 94%, STR = 6%
Recurrence rate = 7.4%
Mean follow-up: 49 months
Complications (n): 0 deaths, 17 CSF leaks, 0 meningitis cases, 13 superficial wound infections
The authors conclude that the endoscope provides improved visualization, smaller craniotomies, decreased operative time (193 minutes), and decreased complications.
|
|
Chovanec et al30 (2013)
|
Prospective patient choice of consecutive patients, 2008–2010.
89 consecutive cases, patient selected MS (n = 44, mean size 2.8 cm, mean age 45 years) or EA (n = 39, mean size 2.6 cm, mean age 47 years).
|
N/A
|
| |
EA
|
MS
|
|
Good FN outcome
|
80%
|
58%
|
|
Useful hearing preservation
|
21%
|
2%
|
|
Gross total resection
|
100%
|
100%
|
|
Recurrence
|
0%
|
2%
|
|
CSF leak
|
8%
|
20%
|
|
Wound infection
|
0%
|
0%
|
|
Mean follow-up
|
26 months
|
28 months
|
The authors conclude that endoscopic resection appears to improve surgical outcome and reduce CSF leaks. It should be noted that the authors used the endoscope as an adjunct to the procedure and did not perform the operation completely with the endoscope. They note in 7 EA cases they believed they achieved complete resections; however, when the endoscope was inserted they had found residual tumor.
|
|
Goksu et al31 (2005)
|
Retrospective case series, 1996–2002. 60 patients all retrosigmoid with EA. Mean size = 2.3 cm (range 0.6–5.0 cm)
|
N/A
|
24% useful hearing preservation
63% facial preservation (good facial outcome)
Resection (GTR, STR) not reported
Recurrence rate = 0%
Mean follow-up: not reported
Complications (n): 0 deaths, 8 CSF leaks, 1 meningitis case, 0 superficial wound infections
The authors conclude that the endoscope does offer improved visualization, especially of the fundus/distal IAC. Their hearing preservation or facial nerve outcomes were not improved with endoscope assistance.
|
|
Magnan et al32 (2002)
|
Retrospective case series, 1993–1998.
119 patients treated with retrosigmoid craniotomy with EA. Mean size not reported; however, all 119 tumors were <2.5 cm
|
N/A
|
30% useful hearing preservation
96% facial preservation (good facial outcome)
Resection (GTR, STR): not reported
Recurrence rate = 2.5%
Mean follow-up: not reported
CSF leak rate (n): 10 cases (12%), no deaths
The authors conclude that in small- to medium-sized tumors endoscope assistance aids in dissection of tumor that is distal in the IAC that is difficult to see because of preservation of the labyrinth. They state using an endoscope limits the direct line of sight issues used for this drilling and allows a smaller craniotomy.
|
|
Kumon et al38 (2012)
|
Retrospective case series, years: “from January 2000.” Comparing EA (28) and fully microscopic (43) retrosigmoid approach for extent of residual IAC tumor. Decision for each approach was left to surgeon’s discretion. EA tumors were smaller compared to fully microscopic cases (21.4 mm vs 26.0 mm).
|
N/A
|
No statistical difference in rates of facial nerve function, hearing preservation.
More likely to have residual IAC tumor identified in the microscope-only group compared with the endoscopic-assisted group; however, no difference in actual recurrence between groups at a mean follow-up of 55.6 and 60.9 months
Facial nerve outcomes used the Yanagihara system, therefore difficult to compare to most literature.
Useful postoperative hearing was 50% for both groups.
Rate of CSF leak not described.
The authors conclude that “(for complete tumor removal) the benefit of endoscope-assistance microsurgery was shown for those patients whose tumors extended beyond the mid-portion of the IAC but did not reach the fundus.”
Significant selection bias in cases, and retrospective in nature. Also, the conclusions only state that for a specific group of patients, the endoscope helped for complete tumor removal in the IAC. Overall, shows some (weak) evidence that visualization of the IAC is better, but no other advantages.
|
|
Wackym et al39 (1999)
|
Multicenter retrospective case series, only including EA. Looking at where the endoscope helped. Years June 1993–January 1999. 78 patients via retrosigmoid, translabyrinthine, and middle fossa. Mean tumor size was not reported. Reported in groups.
|
N/A
|
2 CSF leaks through wound, no rhinorrhea
FN function and recurrence not reported
After microscope work, the endoscope identified residual tumor at the fundus in 11 patients
25 patients had air cells that were caught with the endoscope after not being seen with the microscope.
No added complications and minimal time increase with endoscope.
Evidence is poor, but basically the authors subjectively feel that visualization is better with the endoscope with no added morbidity. Unknown if recurrence rate or CSF leak rate is actually lower since no comparison group.
|
|
Gerganov et al33 (2010)
|
Prospective case series with historical controls. Years not reported. 30 patients with EA compared to 50 MS controls treated through a retrosigmoid craniotomy. MS (mean size 2.2 cm, mean age 46 years) or EA (mean size 2.0 cm, mean age 47 years).
|
N/A
|
| |
EA
|
MS
|
|
Good FN outcome
|
70%
|
66%
|
|
Useful hearing preservation
|
57%
|
52%
|
|
Gross total resection
|
100%
|
98%
|
|
Recurrence
|
NR
|
NR
|
|
CSF leak
|
3%
|
6%
|
Follow-up not reported
The authors looked primarily at the electrophysiologic outcome of surgery between the 2 techniques because it has been reported that the heating of the endoscope would cause more nerve damage. They concluded the application of the endoscope is a safe technique that does not lead to heat-related issues and is valuable for assessing completeness of resection and air cells that may lead to a CSF leak.
|
CSF, cerebrospinal fluid; EA, endoscope-assisted microsurgery; FN, facial nerve; GTR, gross total resection; HB, House–Brackmann; IAC, internal auditory canal; IV, intravenous; MS, microsurgery; N/A, not applicable; PO, per os; STR, subtotal resection; VS, vestibular schwannoma.
Table 9. Novel molecular targets
Conclusion
|
Author (Year)
|
Study Description
|
Data Class
|
|
Agnihotri et al35 (2014)
|
Multi-institution retrospective study (Toronto/Germany). Retrospective case series of 49 schwannomas with 7 normal comparisons. 36 patients with sporadic VS and 13 with NF2.
|
NA
|
No gene expression differences were seen between the two. The results of this study show that VSs are largely molecularly homogenous at the transcript level and suggest that targeting of the PI3K/AKT/mTOR pathway using novel dual inhibitors may provide new therapeutic intervention for a substantial majority of schwannoma patients. This microarray and clustering analysis revealed upregulated signaling pathways included known alterations in VS, such as PI3K signaling and mTOR signaling (P < .001), and other cancer signaling pathways, including RAC signaling, CDC42 signaling, and amyloid processing (P < .05). Micro-RNA processing, ROS scavenging, and MYCN signaling were significantly downregulated (P < .05). As the PI3K/AKT/mTOR signaling networks were most consistently upregulated in the study population, the authors hypothesize this pathway may be a target for molecular therapy of these tumors.
|
mTOR, mammalian target of rapamycin; NF2, neurofibromatosis type 2; ROS, reactive oxygen species; VS, vestibular schwannoma.
Table 10.Ongoing trials registered with clinicaltrials.gov*
|
NCT Number
|
Title
|
Start Date
|
|
NCT02282917
|
Exploratory evaluation of AR-42 histone deacetylase inhibitor in the treatment of vestibular schwannoma
|
10/1/2014
|
|
NCT00863122
|
Concentration and activity of lapatinib in vestibular schwannomas
|
6/1/2009
|
|
NCT02379754
|
Gentamicin treatment prior to schwannoma surgery-residual function
|
1/1/2015
|
|
NCT02415257
|
Gentamicin treatment prior to schwannoma surgery-no residual function
|
4/1/2015
|
|
NCT01767792
|
Phase 2 study of bevacizumab in children and young adults with NF 2 and progressive vestibular schwannomas
|
5/1/2013
|
|
NCT02275325
|
Preoperative vestibular rehabilitation effectiveness after vestibular schwannoma surgery
|
1/1/2015
|
|
NCT02129647
|
Study of axitinib in patients with neurofibromatosis type 2 and progressive vestibular schwannomas
|
4/1/2014
|
*Major ongoing randomized clinical trials pertaining to the use of emerging therapies that evaluate treatment comparisons addressed by this guideline paper for the management of vestibular schwannomas.
References
1. Kastner M, Wilczynski NL, Walker-Dilks C, McKibbon KA, Haynes B. Age-specific search strategies for Medline. J Med Internet Res 2006;8(4):e25.
2. Haynes RB, McKibbon KA, Wilczynski NL, Walter SD, Werre SR, Hedges T. Optimal search strategies for retrieving scientifically strong studies of treatment from Medline: analytical survey. BMJ 2005;330(7501):1179.
3. Montori VM, Wilczynski NL, Morgan D, Haynes RB, Hedges T. Optimal search strategies for retrieving systematic reviews from Medline: analytical survey. BMJ 2005;330(7482):68.
4. Wong SS, Wilczynski NL, Haynes RB. Comparison of top-performing search strategies for detecting clinically sound treatment studies and systematic reviews in MEDLINE and EMBASE. J Med Libr Assoc 2006;94(4):451-455.
5. Zhang L, Ajiferuke I, Sampson M. Optimizing search strategies to identify randomized controlled trials in MEDLINE. BMC Med Res Methodol 2006;6:23.
6. Topfer LA, Parada A, Menon D, Noorani H, Perras C, Serra-Prat M. Comparison of literature searches on quality and costs for health technology assessment using the MEDLINE and EMBASE databases. Int J Technol Assess Health Care 1999;15(2):297-303.
7. Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound prognostic studies in MEDLINE: an analytic survey. BMC Med 2004;2:23.
8. Wilczynski NL, Haynes RB, Hedges T. EMBASE search strategies achieved high sensitivity and specificity for retrieving methodologically sound systematic reviews. J Clin Epidemiol 2007;60(1):29-33.
9. Alanin MC, Klausen C, Caye-Thomasen P, et al. The effect of bevacizumab on vestibular schwannoma tumour size and hearing in patients with neurofibromatosis type 2. Eur Arch Otorhinolaryngol 2015;272(12):3857-3860.
10. Plotkin SR, Merker VL, Halpin C, et al. Bevacizumab for progressive vestibular schwannoma in neurofibromatosis type 2: a retrospective review of 31 patients. Otol Neurotol 2012;33(6):1046-1052.
11. Plotkin SR, Stemmer-Rachamimov AO, Barker 2nd FG, et al. Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med 2009;361(4):358-367.
12. Bush ML, Oblinger J, Brendel V, et al. AR42, a novel histone deacetylase inhibitor, as a potential therapy for vestibular schwannomas and meningiomas. Neurooncology 2011;13(9):983-999.
13. Jacob A, Oblinger J, Bush ML, et al. Preclinical validation of AR42, a novel histone deacetylase inhibitor, as treatment for vestibular schwannomas. Laryngoscope 2012;122(1):174-189.
14. Altuna X, Lopez JP, Yu MA, et al. Potential role of imatinib mesylate (Gleevec, STI-571) in the treatment of vestibular schwannoma. Otol Neurotol 2011;32(1):163-170.
15. Ammoun S, Cunliffe CH, Allen JC, et al. ErbB/HER receptor activation and preclinical efficacy of lapatinib in vestibular schwannoma. Neurooncology 2010;12(8):834-843.
16. Karajannis MA, Legault G, Hagiwara M, et al. Phase II trial of lapatinib in adult and pediatric patients with neurofibromatosis type 2 and progressive vestibular schwannomas. Neurooncology 2012;14(9):1163-1170.
17. Plotkin SR, Halpin C, McKenna MJ, Loeffler JS, Batchelor TT, Barker 2nd FG. Erlotinib for progressive vestibular schwannoma in neurofibromatosis 2 patients. Otol Neurotol 2010;31(7):1135-1143.
18. Karajannis MA, Legault G, Hagiwara M, et al. Phase II study of everolimus in children and adults with neurofibromatosis type 2 and progressive vestibular schwannomas. Neurooncology 2014;16(2):292-297.
19. Kandathil CK, Dilwali S, Wu CC, et al. Aspirin intake correlates with halted growth of sporadic vestibular schwannoma in vivo. Otol Neurotol 2014;35(2):353-357.
20. de Vries M, Briaire-de Bruijn I, Malessy MJA, de Bruine SFT, van der Mey AGL, Hogendoorn PCW. Tumor-associated macrophages are related to volumetric growth of vestibular schwannomas. Otol Neurotol 2013;34(2):347-352.
21. Strauss C, Romstock J, Fahlbusch R, Rampp S, Scheller C. Preservation of facial nerve function after postoperative vasoactive treatment in vestibular schwannoma surgery. Neurosurgery 2006;59(3):577-584.
22. Scheller C, Richter HP, Engelhardt M, Koenig R, Antoniadis G. The influence of prophylactic vasoactive treatment on cochlear and facial nerve functions after vestibular schwannoma surgery: a prospective and open-label randomized pilot study. Neurosurgery 2007;61(1):92-97.
23. Scheller C, Wienke A, Wurm F, et al. Neuroprotective efficacy of prophylactic enteral and parenteral nimodipine treatment in vestibular schwannoma surgery: a comparative study. J Neurol Surg A Cent Eur Neurosurg 2014;75(4):251-258.
24. Scheller C, Strauss C, Fahlbusch R, Romstock J. Delayed facial nerve paresis following acoustic neuroma resection and postoperative vasoactive treatment. Zentralbl Neurochir 2004;65(3):103-107.
25. Tjernstrom F, Fransson PA, Kahlon B, et al. Vestibular PREHAB and gentamicin before schwannoma surgery may improve long-term postural function. J Neurol Neurosurg Psychiatry 2009;80(11):1254-1260.
26. Magnusson M, Kahlon B, Karlberg M, Lindberg S, Siesjo P. Preoperative vestibular ablation with gentamicin and vestibular “prehab” enhance postoperative recovery after surgery for pontine angle tumours--first report. Acta Otolaryngol 2007;127(12):1236-1240.
27. Preoperative vestibular rehabilitation effectiveness after vestibular schwannoma surgery. Available at: https://clinicaltrials.gov/ct2/show/NCT02275325?cond=NCT02275325&rank=1. Accessed November 24, 2017.
28. Kabil MS, Shahinian HK. A series of 112 fully endoscopic resections of vestibular schwannomas. Minim Invasive Neurosurg 2006;49(6):362-368.
29. Shahinian HK, Ra Y. 527 fully endoscopic resections of vestibular schwannomas. Minim Invasive Neurosurg 2011;54(2):61-67.
30. Chovanec M, Zvĕřina E, Profant O, et al. Impact of video-endoscopy on the results of retrosigmoid-transmeatal microsurgery of vestibular schwannoma: prospective study. Eur Arch Otorhinolaryngol 2013;270(4):1277-1284.
31. Goksu N, Yilmaz M, Bayramoglu I, Aydil U, Bayazit YA. Evaluation of the results of endoscope-assisted acoustic neuroma surgery through posterior fossa approach. ORL J Otorhinolaryngol Relat Spec 2005;67(2):87-91.
32. Magnan J, Barbieri M, Mora R, et al. Retrosigmoid approach for small and medium-sized acoustic neuromas. Otol Neurotol 2002;23(2):141-145.
33. Gerganov VM, Giordano M, Herold C, Samii A, Samii M. An electrophysiological study on the safety of the endoscope-assisted microsurgical removal of vestibular schwannomas. Eur J Surg Oncol 2010;36(4):422-427.
34. Alobaid A, Aref M, Bennardo MR, Farrokhyar F, Reddy K. Facial nerve outcome after vestibular schwannoma resection: a comparative meta-analysis of endoscopic versus open retrosigmoid approach. J Neurol Surgery B Skull Base 2015;76(2):157-162.
35. Agnihotri S, Gugel I, Remke M, et al. Gene-expression profiling elucidates molecular signaling networks that can be therapeutically targeted in vestibular schwannoma. J Neurosurg 2014;121(6):1434-1445.
36. Scheller C, Wienke A, Wurm F, et al. Enteral or parenteral nimodipine treatment: a comparative pharmacokinetic study. J Neurol Surg A Cent Eur Neurosurg 2014;75(2):84-90.
37. Magnusson M, Karlberg M, Tjernstrom F. ‘PREHAB’: vestibular prehabilitation to ameliorate the effect of a sudden vestibular loss. NeuroRehabilitation 2011;29(2):153-156.
38. Kumon Y, Kohno S, Ohue S, et al. Usefulness of endoscope-assisted microsurgery for removal of vestibular schwannomas. J Neurol Surg B Skull Base 2012;73(1):42-47.
39. Wackym PA, King WA, Poe DS, et al. Adjunctive use of endoscopy during acoustic neuroma surgery. Laryngoscope 1999;109(8):1193-1201.
© Congress of Neurological Surgeons
Source: Neurosurgery, February 2018