Guidelines on the Evaluation and Treatment of Patients with Thoracolumbar Spine Trauma
8. Nonoperative Care
download pdf Neurosurgery, 2018
Sponsored by: Congress of Neurological Surgeons and the Section on Disorders of the Spine and Peripheral Nerves in collaboration with the Section on Neurotrauma and Critical Care
Endorsed by: The Congress of Neurological Surgeons (CNS) and the American Association of Neurological Surgeons (AANS)
Daniel J. Hoh, MD,1 Sheeraz Qureshi, MD, MBA,2 Paul A. Anderson, MD,3 Paul M. Arnold, MD,4 John H. Chi, MD, MPH,5 Andrew T. Dailey, MD,6 Sanjay S. Dhall, MD,7 Kurt M. Eichholz, MD,8 James S. Harrop, MD,9 Craig H. Rabb, MD,10 P. B. Raksin, MD,11 Michael G. Kaiser, MD,12 and John E. O’Toole, MD, MS13
1. Lillian S. Wells Department of Neurological Surgery, University of Florida, Gainesville, Florida
2. Department of Orthopaedic Surgery, Weill Cornell Medical College, New York, New York
3. Department of Orthopedics and Rehabilitation, University of Wisconsin, Madison, Wisconsin
4. Department of Neurosurgery, University of Kansas School of Medicine, Kansas City, Kansas
5. Department of Neurosurgery, Harvard Medical School, Brigham and Women’s Hospital, Boston, Massachusetts
6. Department of Neurosurgery, University of Utah, Salt Lake City, Utah
7. Department of Neurological Surgery, University of California, San Francisco, San Francisco, California
8. St. Louis Minimally Invasive Spine Center, St. Louis, Missouri
9. Departments of Neurological Surgery and Orthopedic Surgery, Thomas Jefferson University, Philadelphia, Pennsylvania
10. Department of Neurosurgery, University of Utah, Salt Lake City, Utah
11. Division of Neurosurgery, John H. Stroger, Jr. Hospital of Cook County and Department of Neurological Surgery, Rush University Medical Center, Chicago, Illinois
12. Department of Neurosurgery, Columbia University, New York, New York
13. Department of Neurological Surgery, Rush University Medical Center, Chicago, Illinois
Correspondence:
Daniel J. Hoh, MD
Lillian S. Wells Department of Neurological Surgery
University of Florida
Box 100265
Gainesville, FL 32610
Email:Daniel.Hoh@neurosurgery.ufl.edu
Keywords: Bracing, nonoperative care, orthosis, lumbar burst fracture, thoracic burst fracture
Abbreviations
CT - Computed tomography
ODI - Oswestry Disability Index
RMDQ - Roland Morris Disability Questionnaire
SF-36 - Short Form-36 Health Survey
TLSO - Thoracolumbosacral orthosis
VAS - Visual analog scale
ABSTRACT
Background: Thoracic and lumbar burst fractures in neurologically intact patients are considered to be inherently stable and responsive to nonsurgical management. There is a lack of consensus regarding the optimal conservative treatment modality. The question remains whether external bracing is necessary versus mobilization without a brace after these injuries.
Objective: The purpose of this evidence-based clinical practice guideline is to determine if the use of external bracing improves outcomes compared to no brace for neurologically intact patients with thoracic or lumbar burst fractures.
Methods: A systematic review of the literature was performed using the National Library of Medicine PubMed database and the Cochrane Library for studies relevant to thoracolumbar trauma. Clinical studies specifically comparing external bracing to no brace for neurologically intact patients with thoracic or lumbar burst fractures were selected for review.
Results: Three studies out of 1137 met inclusion criteria for review. One randomized controlled trial (level I) and an additional randomized controlled pilot study (level II) provided evidence that both external bracing and no brace equally improve pain and disability in neurologically intact patients with burst fractures. There was no difference in final clinical and radiographic outcomes between patients treated with an external brace versus no brace. One additional level IV retrospective study demonstrated equivalent clinical outcomes for external bracing versus no brace.
Conclusion: This evidence-based guideline provides a grade B recommendation that management either with or without an external brace is an option given equivalent improvement in outcomes for neurologically intact patients with thoracic and lumbar burst fractures. The decision to use an external brace is at the discretion of the treating physician, as bracing is not associated with increased adverse events compared to no brace.
RECOMMENDATIONS
|
Question
|
|
Does the use of external bracing improve outcomes in the nonoperative treatment of neurologically intact patients with thoracic and lumbar burst fractures?
|
|
Recommendation
|
|
The decision to use an external brace is at the discretion of the treating physician, as the nonoperative management of neurologically intact patients with thoracic and lumbar burst fractures either with or without an external brace produces equivalent improvement in outcomes. Bracing is not associated with increased adverse events compared to not bracing.
|
|
Strength of Recommendation: Grade B
|
INTRODUCTION
Goals and Rationale
This clinical guideline was created to improve patient care by outlining the appropriate information gathering and decision-making processes involved in the evaluation and treatment of patients with thoracolumbar spine trauma. The surgical management of these patients often takes place under a variety of circumstances and by various clinicians. This guideline was created as an educational tool to guide qualified physicians through a series of diagnostic and treatment decisions to improve the quality and efficiency of care.
Burst fractures are a common injury pattern following trauma to the thoracic and lumbar spine. They are characterized by axial compression of the vertebral body without concomitant shear, rotation, or translational injury.1,2 Primary loading failure of the anterior and middle spinal column can result in vertebral body height loss, anterior wedging with kyphosis, and fracture comminution with retropulsion of posterior body wall fragments into the canal.3-6 The inherent stability of a thoracic or lumbar burst fracture is typically characterized by the severity of these features, as well as the presence of neurologic deficit.7-12 Burst fractures with significant vertebral collapse, angulation, canal compromise, or associated neurologic deficit are generally considered to be unstable and necessitate surgical intervention.13-17
Conversely, burst fractures without neurologic deficit are thought to be relatively stable.18-21 Reports of successful nonoperative treatment of burst fractures in neurologically intact patients point to the overall stability of this particular injury pattern.22-27 Nonoperative treatment options include bed rest, external orthosis with a brace or plaster cast, or early mobilization without orthosis.28-33 Reported benefit of each of these modalities suggests that burst fractures without neurologic deficit are likely inherently stable, which begs the question as to which nonoperative approach is best. Managing patients with bed rest prolongs hospitalization and potentially incurs added morbidity related to restricted mobilization.34,35 Treatment with an external orthosis, however, requires strict patient compliance, is costly, and may delay patient mobilization if access or availability of a properly fitted brace is limited.36-38
Given these issues, the benefit of external bracing compared to no brace in the nonoperative treatment of patients with neurologically intact burst fractures with respect to neurologic function, pain, and disability is a clinically relevant question. Alternatively, proven safety and efficacy of management without a brace may optimize resource utilization, encourage early mobilization, enhance patient engagement, and provide additional viable options at the discretion of the treating physician. Therefore, the purpose of this evidence-based guideline is to address the question of whether external bracing improves outcomes versus no brace in the nonoperative treatment of neurologically intact patients with thoracic or lumbar burst fractures.
Methods
The guidelines task force initiated a systematic review of the literature relevant to the diagnosis and treatment of patients with thoracolumbar trauma. Through objective evaluation of the evidence and transparency in the process of making recommendations, this evidence-based clinical practice guideline was developed for the diagnosis and treatment of adult patients with thoracolumbar injury. These guidelines are developed for educational purposes to assist practitioners in their clinical decision-making processes. Additional information about the methods used in this systematic review can be found in the introduction and methodology chapter.
Literature Search
The task force members identified search terms/parameters, and a medical librarian implemented the literature search, consistent with the literature search protocol (see Appendix I), using the National Library of Medicine PubMed database and the Cochrane Library (which included the Cochrane Database of Systematic Reviews, the Database of Abstracts of Reviews of Effect, the Cochrane Central Register of Controlled Trials, the Health Technology Assessment Database, and the National Health Service Economic Evaluation Database) for the period from January 1, 1946 to March 31, 2015, using the search strategies provided in Appendix I.
RESULTS
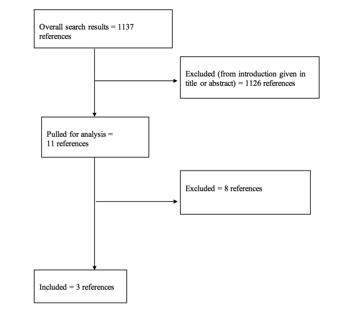
The literature search yielded 1137 abstracts. Task force members reviewed all abstracts yielded from the literature search and identified the literature for full-text review and extraction, addressing the clinical questions, in accordance with the literature search protocol (Appendix I). Task force members identified the best research evidence available to answer the targeted clinical questions. When level I, II, and/or III literature was available to answer specific questions, the task force did not review level IV studies.
The task force selected 11 full-text articles for review. Of these, 8 were rejected for not meeting inclusion criteria or for being off topic. Three were selected for inclusion in this systematic review (Appendix II).
Inclusion/Exclusion Criteria
Articles were retrieved and included only if they met specific inclusion/exclusion criteria. These criteria were also applied to articles provided by guideline task force members who supplemented the electronic database searches with articles from their own files. To reduce bias, these criteria were specified before conducting the literature searches.
Articles that do not meet the following criteria were, for the purposes of this evidence-based clinical practice guideline, excluded. To be included as evidence in the guideline, an article had to be a report of a study that:
- Investigated patients with thoracolumbar injuries;
- Included patients ≥18 years of age;
- Enrolled ≥80% of thoracolumbar injuries (studies with mixed patient populations were included if they reported results separately for each group/patient population);
- Was a full article report of a clinical study;
- Was not an internal medical records review, meeting abstract, historical article, editorial, letter, or commentary;
- Appeared in a peer-reviewed publication or a registry report;
- Enrolled ≥10 patients per arm per intervention (20 total) for each outcome;
- Included only human subjects;
- Was published in or after 1946 through March 31, 2015;
- Quantitatively presented results;
- Was not an in vitro study;
- Was not a biomechanical study;
- Was not performed on cadavers;
- Was published in English;
- Was not a systematic review, meta-analysis, or guideline developed by others*;
- Was a case series (therapeutic study) where higher level evidence exists
Rating Quality of Evidence
The guideline task force used a modified version of the North American Spine Society’s (NASS) evidence-based guideline development methodology. The NASS methodology uses standardized levels of evidence (Appendix III) and grades of recommendation (Appendix IV) to assist practitioners in easily understanding the strength of the evidence and the recommendations within the guidelines. The levels of evidence range from level I (high quality randomized controlled trial) to level IV (case series). Grades of recommendation indicate the strength of the recommendations made in the guideline based on the quality of the literature. Levels of evidence have specific criteria and are assigned to studies before developing recommendations. Recommendations are then graded based upon the level of evidence. To better understand how levels of evidence inform the grades of recommendation and the standard nomenclature used within the recommendations, see Appendix IV.
Guideline recommendations were written using a standard language that indicates the strength of the recommendation. “A” recommendations indicate a test or intervention is “recommended”; “B” recommendations “suggest” a test or intervention; “C” recommendations indicate a test or intervention or “is an option.” “Insufficient evidence” statements clearly indicate that “there is insufficient evidence to make a recommendation for or against” a test or intervention. Task force consensus statements clearly state that “in the absence of reliable evidence, it is the task force’s opinion that” a test or intervention may be considered. Both the levels of evidence assigned to each study and the grades of each recommendation were arrived at by consensus of the workgroup employing up to three rounds of voting when necessary.
In evaluating studies as to levels of evidence for this guideline, the study design was interpreted as establishing only a potential level of evidence. As an example, a therapeutic study designed as a randomized controlled trial would be considered a potential level I study. The study would then be further analyzed as to how well the study design was implemented, and significant shortcomings in the execution of the study would be used to downgrade the levels of evidence for the study’s conclusions (see Appendix V for additional information and criteria).
Revision Plans
In accordance with the Institute of Medicine’s standards for developing clinical practice guidelines and criteria specified by the National Guideline Clearinghouse, the task force will monitor related publications after the release of this document and will revise the entire document and/or specific sections “if new evidence shows that a recommended intervention causes previously unknown substantial harm; that a new intervention is significantly superior to a previously recommended intervention from an efficacy or harms perspective; or that a recommendation can be applied to new populations.”39 In addition, the task force will confirm within 5 years from the date of publication that the content reflects current clinical practice and the available technologies for the evaluation and treatment for patients with thoracolumbar trauma.
DISCUSSION
|
Question
|
|
Does the use of external bracing improve outcomes in the nonoperative treatment of neurologically intact patients with thoracic and lumbar burst fractures?
|
Recommendation
|
|
The decision to use an external brace is at the discretion of the treating physician, as the nonoperative management of neurologically intact patients with thoracic and lumbar burst fractures either with or without an external brace produces equivalent improvement in outcomes. Bracing is not associated with increased adverse events compared to not bracing.
|
|
Strength of Recommendation: Grade B
|
Level I Evidence
One randomized controlled trial evaluated external bracing versus no brace treatment for the nonoperative management of thoracic and lumbar burst fractures in neurologically intact patients (Appendix VI). Bailey et al40 performed a randomized controlled trial from 3 Canadian spine centers to compare functional and quality of life outcomes in patients at 3 months after thoracolumbar burst fracture treated either with or without a thoracolumbosacral orthosis (TLSO) brace. Inclusion criteria were isolated to AO type A3 burst fractures2 between T10 and L3, neurologically intact, <35° kyphosis, within 3 days of injury, and between 16 and 60 years of age. Although patients <18 years of age (and >16 years) may have potentially been included in this study, it is not clear from the reported results. Exclusion criteria included patients who were unable to wear a brace, had already mobilized before enrollment, suffered a pathologic or open fracture, were alcohol or drug users, had previous surgery or injury, or were unable to complete outcome questionnaires.
Patients randomized to a TLSO brace were instructed to wear the brace at all times except in bed for a total of 10 weeks. Patients randomized to no brace were mobilized with the assistance of a physiotherapist, with encouragement to return to normal activities at 8 weeks. The primary outcome was assessed by blinded evaluators at 3 months with the secondary outcome followed up to 2 years. Standardized outcome measures included Roland Morris Disability Questionnaire (RMDQ), Short Form-36 Health Survey (SF-36), visual analog scale (VAS) for back pain, patient satisfaction, and kyphosis.
The study enrolled 110 patients with 96 patients completing 3 months of follow-up (87.3%). Forty-seven patients were enrolled in the brace group, and 49 in the no brace group. Eleven percent of patients in the brace group admitted to incomplete compliance. No patients in the no brace group crossed over to brace therapy. Four patients in the brace group (4.2%) and 2 in the no brace group (2.1%) eventually underwent surgical treatment due to neurologic deficit or disabling pain. Significant improvement in RMDQ was observed in both the brace and no brace cohorts at all time points up to 6 months postinjury, after which improvement leveled off (p < .001). In addition, an overall benefit was found for VAS and SF-36 at most time points compared to baseline up to 6 months postinjury. At the primary endpoint of 3 months postinjury, there was no significant difference in RMDQ, VAS, SF-36 patient satisfaction, kyphosis, or length of stay between cohorts, which remained so for later secondary time points. Limitations of this study are that the investigators did not report a standardized diagnostic method, and the lack of subject blinding due to the inherent nature of either wearing or not wearing a brace. Otherwise, this study by Bailey et al40 provides level I evidence that treatment with or without a brace results in equivalent clinical outcomes for neurologically intact thoracic and lumbar burst fractures.
Level II Evidence
Shamji et al41 performed a randomized controlled pilot study at 2 centers to compare nonoperative treatment with or without a brace for neurologically intact thoracolumbar burst fractures. Primary outcomes included radiographic spinal deformity, pain, and disability at 6 months postinjury. Inclusion criteria were acute burst fracture between T10 and L4 diagnosed by computed tomography without neurologic deficit. Exclusion criteria were prior surgery or injury, inability to complete clinical follow-up, and age <18 years old. Patients randomized to TLSO treatment were fitted with a customized brace with instructions to wear the brace for 3 months whenever they were out of bed. Patients randomized to no brace were mobilized after 24 hours of injury. Primary outcome measure was kyphosis measured by sagittal Cobb angle and vertebral body height on standing x-ray at 6 months postinjury, as well as VAS, Oswestry Disability Index, and SF-36. Radiographic evaluation was performed by 2 blinded observers.
The study enrolled 23 patients with 12 randomized to brace and 11 to no brace. There was 100% follow-up at 6 months. No patients crossed over between groups, and none required eventual surgical treatment. Both brace and no brace groups demonstrated significant improvement in VAS at each time point after injury up to 6 months. There was no significant difference in follow-up VAS, ODI, and SF-36 scores between treatment groups. Similarly, there was no significant difference in fractional anterior vertebral body height loss and sagittal Cobb angle between cohorts. The only reported difference between cohorts was a statistically significant shorter length of stay in those treated without a brace. As in the study by Bailey et al,40 the subjects in this randomized controlled trial were not blinded to type of treatment. This randomized pilot study was graded as level II evidence due to small cohort sizes and a lack of a power analysis. Therefore, this study by Shamji et al41 provides level II evidence that treatment without a brace results in similar radiographic and clinical outcomes as external bracing for neurologically intact thoracolumbar burst fractures.
Level IV Evidence
There is 1 level IV study that provides a lower level of evidence comparing external bracing versus no brace for the nonoperative treatment of thoracic and lumbar burst fractures.
Post et al42 performed a retrospective study of functional outcomes in neurologically intact patients who were treated conservatively at a single center for thoracolumbar fractures. Inclusion criteria were AO type A fractures between T10 and L4 without neurologic deficit. Exclusion criteria were prior spinal disorders, pathological fracture, and inability to complete clinical follow-up. Eighty-one patients were identified and contacted by letter for participation. Thirty-three patients ultimately participated in the study at a mean 5.3 years (range 3–8 years) of follow-up. The decision to manage either with or without a brace was determined by the treating physician at initial presentation. Typically, fractures that demonstrated characteristics more suggestive of instability (eg, significant anterior wedging) were treated with a brace, whereas those that were more stable appearing were managed without a brace. Eighteen patients were treated with a brace for 6 months both day and nighttime, followed by 3 months during the day only, for a total of 9 months. Fifteen patients were treated without a brace. Outcome measures included functional assessment with a dynamic lifting test, an ergometry exercise test, and activity restriction. Additional assessment included RMDQ, VAS, and SF-36 survey.
The investigators observed no significant difference in functional outcome between groups based on the dynamic lifting test or ergometry exercise test. Similarly, there was no difference in RMDQ, VAS, or SF-36. Major limitations to this study include: nonconsecutive patient enrollment, no reported standardized diagnostic method, <80% follow-up, potential inclusion of compression and other nonburst AO type A fractures, and heterogeneous cohorts with more unstable fractures undergoing bracing. Therefore, this level III retrospective, comparative study was downgraded to level IV evidence that treatment with or without a brace results in similar functional outcomes for neurologically intact thoracolumbar burst fractures.
Future Research
Several key questions remain regarding the nonoperative management of neurologically intact thoracic and lumbar burst fractures. Optimal protocols with respect to specific activity restrictions, physical therapy, and duration of management have not been standardized. The 2 randomized controlled trials had a primary length of follow-up of 3 or 6 months. The impact of bracing versus no brace on long-term follow-up is relevant and should be studied further.
Both the level I and II studies excluded fractures of the upper and midthoracic and lower lumbar spine. Further studies determining whether the same equivalence of bracing versus no brace for fractures in the rostral thoracic and caudal lumbar spine are necessary. Prior studies have demonstrated broad variability of burst fracture morphology with respect to kyphosis, comminution, and canal compromise. Future research may better elucidate which specific fracture subtypes are better treated surgically or nonoperatively with bracing or no brace. The role of bracing in other neurologically intact nonburst thoracic and lumbar fractures (e.g., compression fractures, Chance fractures, fracture dislocations, and ligamentous injuries) cannot be inferred from this evidence and needs further study. Last, overall cost effectiveness of external bracing versus no brace treatment should be determined. This will ultimately require a complex analysis of costs associated with initial hospitalization, brace, follow-up care, and potential salary loss.
Conclusions
Two randomized controlled studies provided level I and II evidence that neurologically intact patients with thoracic and lumbar burst fractures have equivalent improvement in clinical outcome when treated nonoperatively either with or without a brace. One retrospective comparative study provided additional level IV evidence supporting no difference in outcome between bracing and no brace, albeit downgraded evidence due to major study limitations. It should also be noted that both the level I and II studies did not include burst fractures of the upper and midthoracic or lower lumbar spine. Therefore, one may not be able to extrapolate the findings of these 2 studies to burst fractures of the rostral thoracic or caudal lumbar spine where mechanical forces and inherent stability may be different.
Although outcomes were similar between patients treated nonoperatively with or without a brace, the decision to use an external brace remains an option at the discretion of the treating provider. Overall improvement in clinical outcomes compared to baseline supports both bracing and not bracing as an equivalently effective intervention. Of note, none of the reviewed studies demonstrated adverse events or disadvantages associated with bracing. There are numerous factors that may positively influence the decision to treat a thoracic or lumbar burst fracture with an external brace. These include fracture morphology, pain, disability, and compliance with conservative treatment. External bracing may provide needed patient assurance to promote early mobilization and participation in physical therapy. Therefore, the existing evidence supports management either with or without a brace for neurologically intact patients with thoracic or lumbar burst fractures.
Potential Conflicts of Interest
The task force members were required to report all possible conflicts of interest (COIs) prior to beginning work on the guideline, using the COI disclosure form of the AANS/CNS Joint Guidelines Review Committee, including potential COIs that are unrelated to the topic of the guideline. The CNS Guidelines Committee and Guideline Task Force Chairs reviewed the disclosures and either approved or disapproved the nomination. The CNS Guidelines Committee and Guideline Task Force Chairs are given latitude to approve nominations of Task Force members with possible conflicts and address this by restricting the writing and reviewing privileges of that person to topics unrelated to the possible COIs. The conflict of interest findings are provided in detail in the companion introduction and methods manuscript.
Disclaimer of Liability
This clinical systematic review and evidence-based guideline was developed by a multidisciplinary physician volunteer task force and serves as an educational tool designed to provide an accurate review of the subject matter covered. These guidelines are disseminated with the understanding that the recommendations by the authors and consultants who have collaborated in their development are not meant to replace the individualized care and treatment advice from a patient's physician(s). If medical advice or assistance is required, the services of a competent physician should be sought. The proposals contained in these guidelines may not be suitable for use in all circumstances. The choice to implement any particular recommendation contained in these guidelines must be made by a managing physician in light of the situation in each particular patient and on the basis of existing resources.
Disclosures
These evidence-based clinical practice guidelines were funded exclusively by the Congress of Neurological Surgeons and the Section on Disorders of the Spine and Peripheral Nerves in collaboration with the Section on Neurotrauma and Critical Care, which received no funding from outside commercial sources to support the development of this document.
Acknowledgments
The guidelines task force would like to acknowledge the CNS Guidelines Committee for their contributions throughout the development of the guideline and the AANS/CNS Joint Guidelines Review Committee for their review, comments, and suggestions throughout peer review, as well as the contributions of Trish Rehring, MPH, CHES, Senior Manager of Clinical Practice Guidelines for the CNS, and Mary Bodach, MLIS, Guidelines Specialist and Medical Librarian for assistance with the literature searches. Throughout the review process the reviewers and authors were blinded from one another. At this time, the guidelines task force would like to acknowledge the following individual peer reviewers for their contributions: Maya Babu, MD, MBA, Greg Hawryluk, MD, PhD, Steven Kalkanis, MD, Yi Lu, MD, PhD, Jeffrey J. Olson, MD, Martina Stippler, MD, Cheerag Upadhyaya, MD, MSc, and Robert Whitmore, MD.
REFERENCES
1. Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine (Phila Pa 1976) 1983;8:817-831.
2. Magerl F, Aebi M, Gertzbein SD, Harms J, Nazarian S. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J 1994;3:184-201.
3. Boerger TO, Limb D, Dickson RA. Does ‘canal clearance’ affect neurological outcome after thoracolumbar burst fractures? J Bone Joint Surg Br 2000;82:629-635.
4. Dai LY. Remodeling of the spinal canal after thoracolumbar burst fractures. Clin Orthop Relat Res 2001;382:119-123.
5. Fontijne WP, de Klerk LW, Braakman R, et al. CT scan prediction of neurological deficit in thoracolumbar burst fractures. J Bone Joint Surg Br 1992;74:683-685.
6. Dai LY, Jiang LS, Jiang SD. Conservative treatment of thoracolumbar burst fractures: a long-term follow-up results with special reference to the load sharing classification. Spine (Phila Pa 1976) 2008;33:2536-2544.
7. McAfee PC, Yuan HA, Fredrickson BE, Lubicky JP. The value of computed tomography in thoracolumbar fractures. An analysis of one hundred consecutive cases and a new classification. J Bone Joint Surg Am 1983;65:461-473.
8. Hashimoto T, Kaneda K, Abumi K. Relationship between traumatic spinal canal stenosis and neurologic deficits in thoracolumbar burst fractures. Spine (Phila Pa 1976) 1988;13:1268-1272.
9. McCormack T, Karaikovic E, Gaines RW. The load sharing classification of spine fractures. Spine (Phila Pa 1976) 1994;19:1741-1744.
10. Mehta JS, Reed MR, McVie JL, Sanderson PL. Weight-bearing radiographs in thoracolumbar fractures: do they influence management? Spine (Phila Pa 1976) 2004;29:564-567.
11. Willen J, Anderson J, Toomoka K, Singer K. The natural history of burst fractures at the thoracolumbar junction. J Spinal Disord 1990;3:39-46.
12. Hitchon PW, Abode-Iyamah K, Dahdaleh NS, et al. Nonoperative management in neurologically intact thoracolumbar burst fractures: clinical and radiographic outcomes. Spine (Phila Pa 1976) 2016;41:483-489.
13. Dai LY, Wang XY, Jiang LS. Neurologic recovery from thoracolumbar burst fractures: is it predicted by the amount of initial canal encroachment and kyphotic deformity? Surg Neurol 2007;6:232-237.
14. Kim NH, Lee HM, Chun IM. Neurologic injury and recovery in patients with burst fracture of the thoracolumbar spine. Spine (Phila Pa 1976) 1999;24:290-293.
15. Schnee CL, Ansell LV. Selection criteria and outcome of operative approaches for thoracolumbar burst fractures with and without neurological deficit. J Neurosurg 1997;86:48-55.
16. Benson DR, Burkus JK, Montesano PX, Sutherland TB, McLain RF. Unstable thoracolumbar and lumbar burst fractures treated with the AO fixateur interne. J Spinal Disord 1992;5:335-343.
17. Denis F, Armstrong GW, Searls K, Matta L. Acute thoracolumbar burst fractures in the absence of neurologic deficit. A comparison between operative and nonoperative treatment. Clin Orthop Relat Res 1984;189:142-149.
18. James KS, Wenger KH, Schlegel JD, Dunn HK. Biomechanical evaluation of the stability of thoracolumbar burst fractures. Spine (Phila Pa 1976) 1994;19:1731-1740.
19. Holdsworth F. Fractures, dislocations, and fracture-dislocations of the spine. J Bone Joint Surg Am 1970;52:1534-1551.
20. Moller A, Hasserius R, Redlund-Johnell I, Ohlin A, Karlsson MK. Nonoperatively treated burst fractures of the thoracic and lumbar spine in adults: a 23- to 41-year follow-up. Spine J 2007;7:701-707.
21. Reid DC, Hu R, Davis LA, Saboe LA. The nonoperative treatment of burst fractures of the thoracolumbar junction. J Trauma 1988;28:1188-1194.
22. Cantor JB, Lebwohl NH, Garvey T, Eismont FJ. Nonoperative management of stable thoracolumbar burst fractures with early ambulation and bracing. Spine (Phila Pa 1976) 1993;18:971-976.
23. Mumford J, Weinstein JN, Spratt KF, Goel VK. Thoracolumbar burst fractures. The clinical efficacy and outcome of nonoperative management. Spine (Phila Pa 1976) 1993;18:955-970.
24. de Klerk LW, Fontijne WP, Stijnen T, Braakman R, Tanghe HL, van Linge B. Spontaneous remodeling of the spinal canal after conservative management of thoracolumbar burst fractures. Spine (Phila Pa 1976) 1998;23:1057-1060.
25. Weinstein JN, Collalto P, Lehmann TR. Thoracolumbar “burst” fractures treated conservatively: a long-term follow-up. Spine (Phila Pa 1976) 1988;13:33-38.
26. McEvoy RD, Bradford DS. The management of burst fractures of the thoracic and lumbar spine. Experience in 53 patients. Spine (Phila Pa 1976) 1985;10:631-637.
27. Kinoshita H, Nagata Y, Ueda H, Kishi K. Conservative treatment of burst fractures of the thoracolumbar and lumbar spine. Paraplegia 1993;31:58-67.
28. Chow GH, Nelson BJ, Gebhard JS, Brugman JL, Brown CW, Donaldson DH. Functional outcome of thoracolumbar burst fractures managed with hyperextension casting or bracing and early mobilization. Spine (Phila Pa 1976) 1996;21:2170-2175.
29. Anderson PA. Nonsurgical treatment of patients with thoracolumbar fractures. Instr Course Lect 1995;44:57-65.
30. Alanay A, Yazici M, Acaroglu E, Turhan E, Cila A, Surat A. Course of nonsurgical management of burst fractures with intact posterior ligamentous complex: an MRI study. Spine (Phila Pa 1976) 2004;29:2425-2431.
31. Tropiano P, Huang RC, Louis CA, Poitout DG, Louis RP. Functional and radiographic outcome of thoracolumbar and lumbar burst fractures managed by closed orthopaedic reduction and casting. Spine (Phila Pa 1976) 2003;28:2459-2465.
32. Stadhouder A, Buskens E, Vergroesen DA, Fidler MW, de Nies F, Oner FC. Nonoperative treatment of thoracic and lumbar spine fractures: a prospective randomized study of different treatment options. J Orthop Trauma 2009;23:588-594.
33. Hitchon PW, Torner JC, Haddad SF, Follett KA. Management options in thoracolumbar burst fractures. Surg Neurol 1998;49:619-626.
34. Jacobs RR, Asher MA, Snider RK. Thoracolumbar spinal injuries. A comparative study of recumbent and operative treatment in 100 patients. Spine (Phila Pa 1976) 1980;5:463-477.
35. Li Y, Hai Y, Li L, Feng Y, Wang M, Cao G. Early effects of vertebroplasty or kyphoplasty versus conservative treatment of vertebral compression fractures in elderly polytrauma patients. Arch Orthop Trauma Surg 2015;135:1633-1636.
36. Sypert GW. External spinal orthotics. Neurosurgery 1987;20:642-649.
37. Krag MH, Fox MJ, Haugh LD. Comparison of three lumbar orthoses using motion assessment during task performance. Spine (Phila Pa 1976) 2003;28:2359-2367.
38. van der Roer N, de Bruyne MC, Bakker FC, van Tulder MW, Boers M. Direct medical costs of traumatic thoracolumbar spine fractures. Acta Orthop 2005;76:662-666.
39. Ransohoff DF, Pignone M, Sox HC. How to decide whether a clinical practice guideline is trustworthy. JAMA 2013;309:139-140.
40. Bailey CS, Urquhart JC, Dvorak MF, et al. Orthosis versus no orthosis for the treatment of thoracolumbar burst fractures without neurologic injury: a multicenter prospective randomized equivalence trial. Spine J 2014;14:2557-2564.
41. Shamji MF, Roffey DM, Young DK, Reindl R, Wai EK. A pilot evaluation of the role of bracing in stable thoracolumbar burst fractures without neurological deficit. J Spinal Disord Tech 2014;27:370-375.
42. Post RB, Keizer HJ, Leferink VJ, van der Sluis CK. Functional outcome 5 years after non-operative treatment of type A spinal fractures. Eur Spine J 2006;15:472-478.
Appendix I. Literature Searches
Search Strategies
PubMed
- Lumbar vertebrae [MeSH] OR Thoracic vertebrae [MeSH]
- Spinal Injuries [MeSH] OR Spinal Cord Injuries [MeSH]
- #1 AND #2
- Thoracolumbar [TIAB] OR thoraco-lumbar [TIAB] OR thoraco lumbar [TIAB] OR burst [Title]
- Injur* [TIAB] OR trauma* [TIAB] OR fractur* [TIAB] OR dislocation* [TIAB]
- #4 AND #5
- Lumbar vertebrae/injuries [MeSH] OR Thoracic vertebrae/injuries [MeSH] (3150 results)
- #3 OR #6 OR #7
- Braces [MeSH] OR Casts, Surgical [MeSH] OR Bed rest [MeSH] OR Physical Therapy Modalities[MeSH] OR Rehabilitation [MeSH] OR rehabilitation[SH]
- drug therapy[sh] OR Analgesics[mh] OR analgesics[pa] OR "Muscle Relaxants, Central"[mh] OR Steroids[mh] OR Glucocorticoids[mh] OR Glucocorticoids[pa]
- Brace OR braces OR bracing OR orthos* OR orthotic* OR cast OR casts OR casting OR TSLO [TIAB]
- bed rest OR bedrest OR “physical therapy” OR physiotherap* OR rehabilitation [TIAB]
- NSAID[tiab] OR opioid*[tiab] OR (muscle[tiab] AND relax*[tiab]) OR acetaminophen[tiab] OR naproxen[tiab] OR ibuprofen[tiab] OR hydrocodone[tiab] OR oxycodone[tiab] OR oxycontin[tiab] OR morphine[tiab] OR benzodiazepine*[tiab] OR tramadol[tiab] OR steroid*[tiab] OR prednisone[tiab] OR solumedrol[tiab] OR fentanyl[tiab] OR lidoderm[tiab] OR aspirin[tiab] OR codeine[tiab] OR drug* [TIAB] OR medication* [TIAB]
- (Conservative[tiab] OR non-operat*[tiab] OR nonoperat*[tiab] OR non-surg*[tiab] OR nonsurg*[tiab]) AND (treatment*[tiab] OR therap*[tiab] OR management[tiab])
- #9 OR #10 OR #11 OR #12 OR #13 OR #14
- #8 AND #15
- (animal [MeSH] NOT human [MeSH]) OR cadaver [MeSH] OR cadaver* [Titl] OR comment [PT] OR letter [PT] OR editorial [PT] OR addresses [PT] OR news [PT] OR “newspaper article” [PT] OR case reports [PT]
- #16 NOT #17
- osteoporosis [MH] OR osteoporotic fractures [MH] OR osteoporo* [TITLE] OR spinal neoplasms [MH] OR tumor* [TITLE] OR tumour* [TITLE] OR malignan* [TITLE]
- #18 NOT #19
- #20 AND English [Lang]
Cochrane Library
- Lumbar vertebrae: MeSH descriptor, explode all trees
- Thoracic vertebrae: MeSH descriptor, explode all trees
- #1 OR #2
- Spinal Injuries: MeSH descriptor
- Spinal Cord Injuries: MeSH descriptor
- #4 OR #5
- #3 AND #6
- (Thoracolumbar OR thoraco-lumbar OR thoraco lumbar OR burst) NEAR/4 (Injur* OR trauma* OR fractur* OR dislocation*):ti,ab,kw
- Lumbar vertebrae/injuries: MeSH descriptor, explode all trees
- Thoracic vertebrae/injuries: MeSH descriptor, explode all trees
- #9 OR #10
- #7 OR #8 OR #11
- mh osteoporosis or mh osteoporotic fractures or mh spinal neoplasms
- osteoporo* or tumor* or malignan*:ti
- #13 OR #14
- #12 NOT #15
Appendix II. Article Inclusions and Exclusions
Included and Excluded Articles Flowchart
Levels of Evidence for Primary Research Questiona
|
Types of studies
|
|
|
Therapeutic studies – Investigating the results of treatment
|
Prognostic studies – Investigating the effect of a patient characteristic on the outcome of disease
|
Diagnostic studies – Investigating a diagnostic test
|
Economic and decision analyses – Developing an economic or decision model
|
|
Level I
|
- High-quality randomized trial with statistically significant difference or no statistically significant difference but narrow confidenceintervals
- Systematic reviewb of level I RCTs (and study results were homogenousc)
|
- High-quality prospective studyd (all patients were enrolled at the same point in their disease with
≥80% follow-up of enrolled patients)
- Systematic reviewb of level I studies
|
- Testing of previously developed diagnostic criteria on consecutive patients (with universally applied reference “gold” standard)
- Systematic reviewb of level I studies
|
- Sensible costs and alternatives; values obtained from many studies; with multiway sensitivity analyses
- Systematic reviewb of level I studies
|
|
Level II
|
- Lesser quality RCT (e.g., ≤80% follow-up, no blinding, or improper randomization)
- Prospectived comparative studye
- Systematic reviewb of level II studies or level I studies with inconsistent results
|
- Retrospectivef study
- Untreated controls from an RCT
- Lesser quality prospective study (e.g., patients enrolled at different points in their disease or
≤80% follow-up)
- Systematic reviewb of level II studies
|
- Development of diagnostic criteria on consecutive patients (with universally applied reference “gold” standard)
- Systematic reviewb of level II studies
|
- Sensible costs and alternatives; values obtained from limited studies; with multiway sensitivity analyses
- Systematic reviewb of level II studies
|
|
Level III
|
- Case control studyg
- Retrospectivef comparative studye
- Systematic reviewb of level III studies
|
|
- Study of non consecutive patients; without consistently applied reference “gold” standard
- Systematic reviewb of level III studies
|
- Analyses based on limited alternatives and costs; and poor estimates
- Systematic reviewb of level III studies
|
|
Level IV
|
Case seriesh
|
Case series
|
- Case-control study
- Poor reference standard
|
- Analyses with no sensitivity analyses
|
RCT, Randomized controlled trial.
aA complete assessment of quality of individual studies requires critical appraisal of all aspects of the study design.
bA combination of results from ≥2 previous studies.
cStudies provided consistent results.
dStudy was started before the first patient enrolled.
ePatients treated one way (e.g., instrumented arthrodesis) compared with a group of patients treated in another way (e.g., unsintrumented arthrodesis) at the same institution.
fThe study was started after the first patient enrolled.
gPatients identified for the study based on their outcome, called “cases” (e.g., pseudoarthrosis) are compared to those who did not have outcome, called “controls” (e.g., successful fusion).
hPatients treated one way with no comparison group of patients treated in another way.
Appendix IV. Linking Levels of Evidence to Grades of Recommendation
|
Grade of Recommendation
|
Standard Language
|
Levels of Evidence
|
|
A
|
Recommended
|
Two or more consistent level I studies
|
|
B
|
Suggested
|
One level I study with additional supporting level II or III studies
|
Two or more consistent level II or III studies
|
|
C
|
Is an option
|
One level I, II, or III study with supporting level IV studies
|
Two or more consistent level IV studies
|
|
Insufficient
(insufficient or conflicting evidence)
|
Insufficient evidence to make recommendation for or against
|
A single level I, II, III, or IV study without other supporting evidence
|
>1 study with inconsistent findingsa
|
aNote that in the presence of multiple consistent studies, and a single outlying, inconsistent study, the Grade of Recommendation will be based on the level of the consistent studies.
Appendix V. Criteria Grading the Evidence
The task force used the criteria provided below to identify the strengths and weaknesses of the studies included in this guideline. Studies containing deficiencies were downgraded one level (no further downgrading allowed, unless so severe that study had to be excluded). Studies with no deficiencies based on study design and contained clinical information that dramatically altered current medical perceptions of topic were upgraded.
- Baseline study design (i.e., therapeutic, diagnostic, prognostic) determined to assign initial level of evidence.
- Therapeutic studies reviewed for following deficiencies:
• Failure to provide a power calculation for an RCT;
• High degree of variance or heterogeneity in patient populations with respect to presenting diagnosis/demographics or treatments applied;
• <80% of patient follow-up;
• Failure to utilize validated outcomes instrument;
• No statistical analysis of results;
• Cross over rate between treatment groups of >20%;
• Inadequate reporting of baseline demographic data;
• Small patient cohorts (relative to observed effects);
• Failure to describe method of randomization;
• Failure to provide flowchart following patients through course of study (RCT);
• Failure to account for patients lost to follow-up;
• Lack of independent post-treatment assessment (e.g., clinical, fusion status, etc.);
• Utilization of inferior control group:
• Historical controls;
• Simultaneous application of intervention and control within same patient.
• Failure to standardize surgical/intervention technique;
• Inadequate radiographic technique to determine fusion status (e.g., static radiographs for instrumented fusion).
- Methodology of diagnostic studies reviewed for following deficiencies:
• Failure to determine specificity and sensitivity;
• Failure to determine inter- and intraobserver reliability;
• Failure to provide correlation coefficient in the form of kappa values.
- Methodology of prognostic studies reviewed for following deficiencies:
• High degree of variance or heterogeneity in patient populations with respect to presenting diagnosis/demographics or treatments applied;
• Failure to appropriately define and assess independent and dependent variables (e.g., failure to use validated outcome measures when available).
Appendix VI. Evidence Tables
|
Author, Year
|
Level of Evidence
|
Task Force Conclusions relative to question and rationale for evidence grading
|
|
Bailey et al,40 2014
|
I
|
This paper provides evidence that nonoperative management without a brace is equivalent to treatment with a thoracolumbosacral orthosis for neurologically intact patients with AO type A3 burst fractures from T10–L3
|
|
Post et al,42 2006
|
IV
|
This paper provides evidence that the use of a brace does not improve functional outcome, disability, or pain compared to no brace in the nonoperative treatment of neurologically intact patients with type A thoracolumbar fractures
|
|
Shamji et al,41 2014
|
II
|
This paper provides evidence that nonoperative treatment with or without a brace results in similar radiographic and clinical outcomes at 6 months in neurologically intact patients with thoracolumbar burst fracture. Treatment without brace results in a shorter length of hospitalization than treatment with a brace
|
© Congress of Neurological Surgeons
Source: Neurosurgery, September 6, 2018