Guidelines for Pediatric Myelomeningocele
4. Closure of Myelomeningocele within 48 Hours to Decrease Infection Risk
Download pdf Neurosurgery, 2019
Sponsored by: Congress of Neurological Surgeons (CNS) and the Section on Pediatric Neurosurgery
Endorsed by: The Congress of Neurological Surgeons (CNS), American Association of Neurological Surgeons (AANS), and Spina Bifida Association (SBA)
Alexandra D. Beier, DO1, Dimitrios C. Nikas, MD2, Nadege Assassi3, David F. Bauer, MD4, Jeffrey P. Blount, MD5, Susan R. Durham, MD, MS6, Ann Marie Flannery, MD7, Paul Klimo Jr., MD8, Catherine McClung-Smith, MD9, Patricia Rehring, MPH10, Mandeep S. Tamber, MD, PhD11, Rachana Tyagi, MD12, Catherine A. Mazzola, MD13
- Division of Pediatric Neurosurgery, University of Florida Health Jacksonville, Jacksonville, Florida
- Division of Pediatric Neurosurgery, Advocate Children's Hospital, Oak Lawn, Illinois
- Department of Surgery, Division of Neurosurgery, Robert Wood Johnson Medical School, New Brunswick, New Jersey
- Department of Surgery, Division of Neurosurgery, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire
- Division of Pediatric Neurosurgery, Department of Neurosurgery, University of Alabama at Birmingham; Children’s of Alabama, Birmingham, Alabama
- The University of Vermont Medical Center, Burlington, VT, USA
- Kids Specialty Center, Women’s & Children’s Hospital, Lafayette, Louisiana
- Semmes-Murphey; Department of Neurosurgery, University of Tennessee Health Science Center; Le Bonheur Children’s Hospital, Memphis, Tennessee
- Department of Neurological Surgery, Palmetto Health USC Medical Group, Columbia, South Carolina
- Congress of Neurological Surgeons, Schaumburg, Illinois
- Division of Pediatric Neurosurgery, British Columbia Children’s Hospital, University of British Columbia, Vancouver, British Columbia, Canada
- Department of Neurosurgery, Mercer University Medical School, Macon, Georgia
- Goryeb Children’s Hospital, Morristown, New Jersey; Rutgers Department of Neurological Surgery, Newark, New Jersey
Correspondence:
Alexandra D. Beier, DO
836 Prudential Drive, Suite 1205
Jacksonville, FL 32207
alexandra.beier@jax.ufl.edu
Abbreviations:
CHLA- Children’s Hospital of Los Angeles
COI- conflicts of interest
KID- Kids’ Inpatient Database
MM- myelomeningocele
SB- spina bifida
ABSTRACT
Background: Appropriate timing for closure of myelomeningocele (MM) varies in the literature. Older studies present 48 hours as the timeframe after which infection complication rates rise.
Objective: The objective of this guideline is to determine if closing the MM within 48 hours decreases the risk of wound infection or ventriculitis.
Methods: The Guidelines Task Force developed search terms and strategies used to search PubMed and Embase for relevant literature published between 1966 and September 2016. Strict inclusion/exclusion criteria were used to screen abstracts and to develop a list of relevant articles for full-text review. Full text articles were then reviewed and when appropriate, included in the evidentiary table. The class of evidence was evaluated, discussed and assigned to each study that met inclusion criteria.
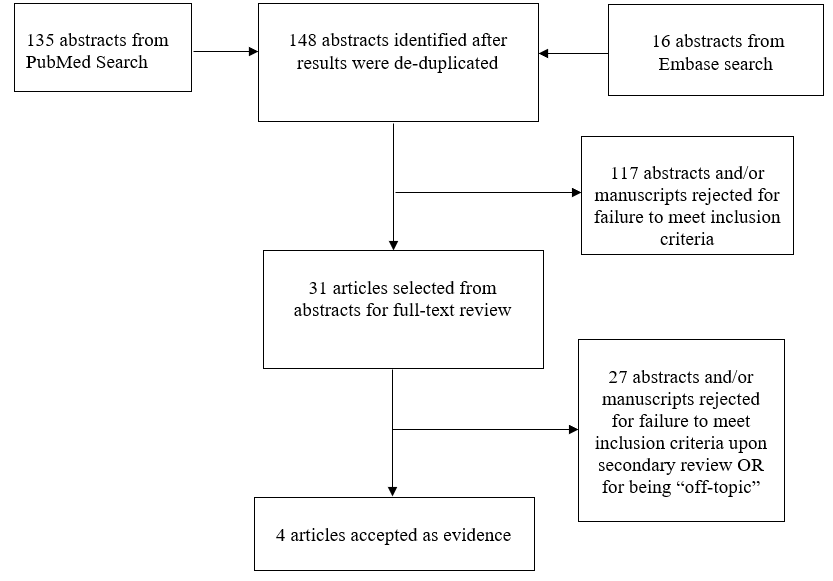
Results: A total of 148 abstracts were identified and reviewed. Thirty-one articles were selected for full text analysis. Only 4 of these studies met inclusion criteria.
Conclusions: There is insufficient evidence that operating within 48 hours decreases risk of wound infection or ventriculitis in 1 Class III study. There is 1 Class III study that provides evidence of global increase in postoperative infection after 48 hours, but is not specific to wound infection or ventriculitis. There is 1 Class III study that provides evidence if surgery is going to be delayed greater than 48 hours, antibiotics should be given.
Keywords: Fetal, infection, meningitis, myelomeningocele, surgery, ventriculitis
RECOMMENDATIONS
PICO Question: In patients born with a myelomeningocele, does closure of the defect within 48 hours reduce the rate of infection?
Target Population: Infants born with a myelomeningocele.
Recommendation(s):
- There is insufficient evidence to confirm that closure of myelomeningoceles within 48 hours decreases the risk of wound infection.
- It is recommended that if myelomeningocele closure is delayed beyond 48 hours, antibiotics should be initiated (Level III).
INTRODUCTION
Rationale
The optimum timing of myelomeningocele (MM) closure has been debated in the literature. The benefits of early closure have been touted to decrease risk of infection. However, there is concern that these infants can be ill and the situation overwhelming to the family, therefore delaying closure may be warranted. In this guideline, the authors address whether the literature clearly shows there to be a decreased risk of infection with closure within 48 hours.
OBJECTIVES
The objective of this guideline is to systematically review the current literature and determine if there is evidence to support closing MM within 48 hours to decrease infection.
METHODS
Writing Group and Question Establishment
The Guidelines Task Force initiated a systematic review of the literature and evidence-based guideline relevant to the diagnosis and treatment of patients with MM. Through objective evaluation of the evidence and transparency in the process of making recommendations, this evidence-based clinical practice guideline was developed for the diagnosis and treatment of patients with MM. These guidelines are developed for educational purposes to assist practitioners in their clinical decision-making processes. Additional information about the methods utilized in this systematic review is provided in the introduction and methodology chapter.
A series of authors for the development of guidelines related to MM were identified and screened for conflict of interest. This group, in turn, agreed on a set of pertinent questions to address the topic at hand, and conducted a systematic review of the literature relevant to MM. The recommendations deliberately eschewed the use of expert opinion, and instead relied strictly on the available literature.
Literature Search
The Guidelines Task Force worked with a research librarian to assist with the formulation of search terms related to MM, time to surgery, complications, and infection used to search PubMed and Embase for relevant literature published between 1966 and September 2016. Co-authors used the article inclusion/exclusion criteria described below to screen 148 abstracts and provide a list of 31 relevant articles for full-text review. Staff compiled the results for review and final approval by all the task force members. All literature identified by searches of the electronic databases was subject to the article inclusion/exclusion criteria listed below. The search strategies used are provided within the methods sections of the topics evaluated below.
Study Selection and Eligibility Criteria
The task force members collaborated with a medical librarian to search PubMed and Embase for the period from 1966 to September 2016 using the search strategies provided in Appendix I. After de-duplication, the literature search yielded 148 abstracts, which were reviewed by the team using the following inclusion and exclusion criteria:
- At least 80% of patients had to be patients with MM and <18 years of age.
- Studies that enrolled >20% of patients with other forms of spina bifida (SB) were excluded.
- Studies that combined the results of patients with other forms of SB were excluded if the study enrolled less than 80% of target patient population.
- Studies that enrolled mixed patient populations were included only if they reported separate results for the target population. The results of the target population were the only results considered as evidence to support our recommendations.
- The study was a full article report of a clinical study.
- The study was not a meeting abstract, editorial, letter, or a commentary.
- Prospective case series had to report baseline values, if applicable.
- Case series studies with non-consecutive enrollment of patients were excluded.
- Studies had to have appeared in a peer-reviewed publication or a registry report.
- Studies had to enroll at least 10 patients for each distinct outcome measured. If it was a comparative study, a minimum enrollment of 5 patients per treatment arm for each outcome was necessary.
- The study involved humans.
- The study was published between January 1966 and September 2016.
- The study presented results quantitatively.
- The study did not involve “in vitro,” “biomechanical,” or results performed on cadavers.
- The study was published in English.
- Papers reporting results of systematic reviews, meta-analyses, or guidelines developed by others were excluded.
- Authors specifically excluded follow-up studies in which a cohort of patients from an initial study were followed in time and separately reported upon in a subsequent publication. This prevented the same patients from being included multiple times in this review.
To reduce bias, these criteria were specified before conducting the literature searches. For the purposes of this evidence review, articles that did not meet the selection criteria are not evidence and not considered as potential evidence to support the clinical recommendations.
Three independent reviewers evaluated each abstract to assess if the article was relevant to our question, and results were compared for agreement by a separate party. Inconsistencies were re-reviewed, and disagreements were resolved by consensus. The authors did not include systematic reviews, guidelines, or meta-analyses conducted by others. These documents were developed using different inclusion criteria than those specified in this guideline. Therefore, they may include studies that do not meet the inclusion criteria specified above. These documents were recalled if their abstract suggested that they might address one of the recommendations, and their bibliographies were searched for additional studies. Of the 31 articles selected, 27 were rejected for not meeting inclusion criteria or for being off-topic. There were 4 studies that met inclusion criteria (see Appendix IV).1-4 See PRISMA Article Flow Chart in Appendix II.
Data Collection Process
The abstracts that met the selection criteria mentioned above were retrieved in full-text form. Each article’s adherence to the selection criteria was confirmed. To determine how the data could be classified, the information in the full-text articles was then evaluated to determine whether they were providing results of therapy or were more centered on diagnostic or prognostic information. Agreement on these assessments and on the salient points regarding the type of study design and objectives, and the conclusions and data classification was then reached by exchanging drafts and comments by e-mail. The information was then used for construction of the evidence tables (see Appendix IV).
Assessment for Risk of Bias
The literature included in the full text review was evaluated for bias utilizing the following criteria: selective result reporting, lack or loss of information over time, publication bias, bias inherent to a retrospective study.
Rating Quality of Evidence
The quality of evidence was rated using an evidence hierarchy for therapeutic studies. Demonstrating the highest degree of clinical certainty, Class I evidence is used to support recommendations of the strongest type, defined as Level I recommendations. Level II recommendations reflect a moderate degree of clinical certainty and are supported by Class II evidence. Level III recommendations denote clinical uncertainty supported by Class III evidence. This hierarchy is shown in Appendix III. Additional information regarding the hierarchy classification of evidence can be located here on the CNS Guidelines methodology page.
Revision Plans
In accordance with the Institute of Medicine’s standards for developing clinical practice guidelines, the task force will monitor related publications following the release of this document and will revise the entire document and/or specific sections “if new evidence shows that a recommended intervention causes previously unknown substantial harm; that a new intervention is significantly superior to a previously recommended intervention from an efficacy or harms perspective; or that a recommendation can be applied to new populations.”5 In addition, the task force will confirm within 5 years from the date of publication that the content reflects current clinical practice and the available technologies for closure of MMC within 48 hours with regards to infection.
RESULTS
Study Selection and Characteristics
The literature available on the topic to discuss if infection is lowered with closure of the MM within 48 hours all stem from retrospective studies. Four studies met criteria for inclusion.1-4
Results of Individual Studies, Discussion of Study Limitations and Risk of Bias
In 1983 Charney et al2 evaluated the time to closure and relation to infection when assessing parent’s emotional support and counseling time before proceeding with informed consent for management of MM. In their series, researchers divided the patient into 3 subgroups based on timing of closure: early (within 48 hours), delayed (3-7 days) and late (>7 days). A total of 10 (out of 96) patients receiving surgery developed ventriculitis. This developed in 9.6% of patients who underwent early surgery, in 12.5% of patients who underwent delayed surgery and in 8.3% of patients who underwent late surgery, with no statistical significance between timing of surgery and infection. This study is limited by its small patient cohort, which is seen in many of these studies. The authors did find that delayed and late surgery infants who received antibiotics were protected from ventriculitis, which was further discussed in Charney et al.2 In 1991, Charney et al1 reviewed 186 surgically treated infants (with overlap from their previous paper) and again divided infants into early (59), delayed (78) and late (22) surgery. Seven percent of patients who underwent early surgery, 6% of patients who underwent delayed surgery and 15% of patients who underwent late surgery all developed ventriculitis, which did not show statistical significance. Antibiotic usage did not decrease the risk of ventriculitis in the early surgery group, however antibiotic usage did decrease the risk with those infants receiving late or delayed surgery, and reached statistical significance, p=0.005.1 Although these are 2 separate papers, we have grouped them as 1 paper for evidence grading, as there is an overlapping population. This collective study was graded as Class III evidence due to the retrospective nature and lack of randomization, power analysis, and infections being the outcome of the study.
More recently, in 2009, a Class III study, Pinto et al3 evaluated surgical outcomes on surgery performed immediately after the birth of the infant (mean time to surgery 90 minutes) compared to historical controls (mean time to surgery 3.9 days). Similar to other studies, the number of total patients was low at 54. The main outcome of the study was to evaluate surgical outcomes, which included infections as well as need for shunting, dehiscence, cerebral spinal fluid leak and neurodevelopmental outcomes.3 The authors found no statistical difference in surgical infection (ventriculitis and meningitis) between the 2 groups (2 patients in each group).
As discussed, due to the infrequent occurrence of MMs, Attenello4 reviewed their series of 95 patients over 10 years at Children’s Hospital of Los Angeles (CHLA) where the median time to surgical closure was performed within 1 day, and compared their results to the 10-year Kids’ Inpatient Database (KID) and Nationwide Inpatient Sample with varying closure times. The CHLA group found 5/95 (5.3%) wound infections with 3/95 (3.2%) patients developed meningitis, but found their data was not powered enough to parse out infection rates based on surgical timing. In comparison, the national cohort found patient who waited >2 days had a 65-88% increased rate of infection compared to those operated on within 24 hours. Procedures performed 1 day after admission compared to same day procedures, did not have a statistically higher infection rate. As the CHLA data and even the previous studies depict, these articles are severely limited by number. The KID database attempted to address this issue, however as this is based on coding, postoperative infection could relate to MM infection, or could be a urinary tract infection, pneumonia, shunt wound infection, etc., rendering it a Class III study.4
DISCUSSION
Postoperative infection after MM closure is a concern, due to the adjacent neural structures and the risk of meningitis. Children with a history of meningitis are at a greater risk of poorer intellectual functioning, with decreases seen in fine motor function, intelligence quotient scores, tests of school behavior, and tests of neuropsychological function.6 Additionally, infection can lead to wound dehiscence and cerebrospinal fluid leak, necessitating further surgery. Therefore, data on surgical timing that would minimize these complications would be very beneficial to these already medically complex children.
Unfortunately, the paucity of randomized controlled studies on the topic of infection based on timing, makes the development of appropriate guidelines difficult. This is likely due to the rarity of MM and average 7-12% infection rate.7 Older studies evaluated the association of the 48 hour surgical time point with decreased infection rate, however they either failed to focus on timing as the main study outcome or were underpowered to show significance.8-11 In conclusion, there does not appear to be definitive evidence that demonstrates closing MM within 48 hours significantly decreases the rate of wound infection and other complications, like meningitis. However, if the MM closure is delayed after 48 hours, then antibiotic therapy should be initiated for the infant to protect against ventriculitis.
Future Research
As discussed, one of the limitations is the infrequent nature of MMs at individual centers. To better address the question of surgical timing, a prospective, multicenter trial will need to occur to ensure enough patients to adequately power the study.
CONCLUSIONS
In conclusion, there is insufficient evidence to support that closing MMs within 48 hours decreases the rate of wound infection and ventriculitis. However, if the MM closure is going to be delayed, antibiotics should be initiated.
Conflict of Interest
The Guidelines Task Force members were required to report all possible conflicts of interest (COIs) prior to beginning work on the guideline, using the COI disclosure form of the AANS/CNS Joint Guidelines Review Committee, including potential COIs that are unrelated to the topic of the guideline. The CNS Guidelines Committee and Guideline Task Force Chair reviewed the disclosures and either approved or disapproved the nomination. The CNS Guidelines Committee and Guideline Task Force Chair are given latitude to approve nominations of Task Force Members with possible conflicts and address this by restricting the writing and reviewing privileges of that person to topics unrelated to the possible COIs. The conflict of interest findings are provided in detail in the companion introduction and methods manuscript.
Disclosures
These evidence-based clinical practice guidelines were funded exclusively by the Congress of Neurological Surgeons, which received no funding from outside commercial sources to support the development of this document.
Disclaimer of Liability
This clinical systematic review and evidence-based guideline was developed by a multidisciplinary physician volunteer task force and serves as an educational tool designed to provide an accurate review of the subject matter covered. These guidelines are disseminated with the understanding that the recommendations by the authors and consultants who have collaborated in their development are not meant to replace the individualized care and treatment advice from a patient's physician(s). If medical advice or assistance is required, the services of a competent physician should be sought. The proposals contained in these guidelines may not be suitable for use in all circumstances. The choice to implement any particular recommendation contained in these guidelines must be made by a managing physician in light of the situation in each particular patient and on the basis of existing resources.
ACKNOWLEDGMENTS
The guidelines task force would like to acknowledge the Congress of Neurological Surgeons Guidelines Committee for their contributions throughout the development of the guideline, the American Association of Neurological Surgeons/Congress of Neurological Surgeons Joint Guidelines Review Committee, as well as the American Academy of Pediatrics, Child Neurology Society and Spina Bifida Association for their review, comments, and suggestions throughout peer review, as well as the contributions of Trish Rehring, MPH, CHES, Senior Manager of Clinical Practice Guidelines for the CNS, and Gretchen Kuntz, MSW, MLIS, for assistance with the literature searches. Throughout the review process, the reviewers and authors were blinded from one another. At this time the guidelines task force would like to acknowledge the following individual peer reviewers for their contributions: Kimon Bekelis, MD; Robin Bowman, MD; Timothy J. Brei, MD; Andrew P. Carlson, MD; John Chi, MD; Mark Dias, MD; Jeffrey Olson, MD; John O’Toole, MD; Michael Partington, MD; Curtis Rozzelle, MD; Krystal Tomei, MD; Jan B. Wollack, MD, PhD.
Appendix I. Literature Search Terms
| PubMed Strategy |
Results |
Embase Strategy |
Results |
Total Results after De-duplication |
| ((((((spina bifida[mh] OR spina bifida[tw])) OR myelomeningocele)) AND (((infant[mh] OR infant[tw])) OR pediatri*[tw])) AND ((complication*[tw]) OR (infection, wound[mh] OR wound infection[tw]))) AND (time factors[mh] OR timing[tw]) |
135 |
(('spinal dysraphism'/exp or 'spina bifida' or 'meningomyelocele') and ('wound infection'/exp or 'wound infection*' or complication*) and ('infant'/exp or infant or pediatr*) and ('time'/exp or timing)) and [embase]/lim not [medline]/lim |
16 |
48 |
Appendix II. PRIMSA Flow Chart for the literature search for closure of myelomeningocele within 48 hours to decrease infection risk
Appendix III: Rating Evidence Quality
Classification of Evidence on Therapeutic Effectiveness
| Class I Evidence Level I Recommendation |
Evidence from one or more well-designed, randomized controlled clinical trial, including overviews of such trials. |
| Class II Evidence Level II Recommendation |
Evidence from one or more well-designed comparative clinical studies, such as non-randomized cohort studies, case-control studies, and other comparable studies, including less well-designed randomized controlled trials. |
| Class III Evidence Level III Recommendation |
Evidence from case series, comparative studies with historical controls, case reports, and expert opinion, as well as significantly flawed randomized controlled trials. |
Appendix IV. Evidence Table
| Article (Alpha by Author) |
Class of Evidence |
Task Force Conclusions relative to question and rationale for evidence grading |
|
Attenello F, 20164
|
Class III |
Retrospective review of a single center institutional 10-year series of MMs, comparing their results to a national cohort. In the national cohort, there was increased risk (65-88%) of postoperative infection in patients who waited 2 or more days for surgery. However, postoperative infection is not specific to only wound infections or meningitis. In the inpatient cohort, there was no significant association between increased wait times and infection, but results were limited by the statistical lack of power. |
|
Charney E; 19832
|
Class III |
Retrospective review of outcomes in MMs. The patients were divided into 3 groups: early (within 48 hours), delayed (within 3-7 days), and late (>7 days). 10/96 patients developed ventriculitis, but there was no statistical significance (9.6% early, 12.5% delayed and 8.3% late surgery). The authors did find patients who had delayed or late surgery did not develop ventriculitis if they were on antibiotics (0% vs 22%, p< .05). |
|
Charney E; 19911
|
Class III |
Charney followed his previous study with another retrospective study evaluating ventriculitis. Development of ventriculitis was not associated with timing of surgical intervention (7% early, 6% delayed, 15% late). However, there is data to support broad spectrum antibiotic prophylaxis is effective in minimizing the risk of ventriculitis among infants receiving surgical intervention after 48 hours: 1% with delayed or late surgery on antibiotics developed ventriculitis compared to the 19% that didn’t receive antibiotics, p=0.005. As there is an overlapping population, they are presented as 1 class of evidence. |
| Pinto F; 20093 |
Class III |
Retrospective review of medical records (31) and prospectively followed patients (23) and assessed infection after immediate surgery. In evaluating infections with immediate surgery (mean time to surgery 90 minutes) compared to historical controls (mean 3.9 days) found no statistical difference in infection rate (9% vs 6%). |
REFERENCES
- Charney EB, Melchionni JB, Antonucci DL. Ventriculitis in newborns with myelomeningocele. Am. J. Dis. Child. Mar 1991;145(3):287-290.
- Charney EB, Sutton LN, Bruce DA, Schut LB. Myelomeningocele newborn management: time for parental decision. Z. Kinderchir. Dec 1983;38(Suppl. II):90-93.
- Pinto FC, Matushita H, Furlan AL, et al. Surgical treatment of myelomeningocele carried out at 'time zero' immediately after birth. Pediatr. Neurosurg. 2009;45(2):114-118.
- Attenello FJ, Tuchman A, Christian EA, et al. Infection rate correlated with time to repair of open neural tube defects (myelomeningoceles): an institutional and national study. Childs Nerv. Syst. Sep 2016;32(9):1675-1681.
- Ransohoff DF, Pignone M, Sox HC. How to decide whether a clinical practice guideline is trustworthy. JAMA. Jan 9 2013;309(2):139-140.
- Grimwood K, Anderson VA, Bond L, et al. Adverse outcomes of bacterial meningitis in school-age survivors. Pediatrics. May 1995;95(5):646-656.
- Schroeder HK, Nunes JC, Madeira L, Moritz JL, Walz R, Linhares MN. Postsurgical infection after myelomeningocele repair: a multivariate analysis of 60 consecutive cases. Clin. Neurol. Neurosurg. Sep 2012;114(7):981-985.
- John W, Sharrard W, Zachary RB, Lorber J, Bruce AM. A Controlled Trial of Immediate and Delayed Closure of Spina Bifida Cystica. Arch. Dis. Child. Feb 1963;38(197):18-22.
- Henry AP, Wood H, Mickel RE. Spina bifida in African and Indian babies. J. Bone Joint Surg. Br. Nov 1974;56-B(4):650-657.
- Fernandez-Serrats AA, Guthkelch AN, Parker SA. The prognosis of open myelocele with a note on a trial of Laurence's operation. Dev. Med. Child Neurol. 1967:Suppl 13:65-74.
- Smyth BT, Piggot J, Forsythe WI, Merrett JD. A controlled trial of immediate and delayed closure of myelomeningocele. J. Bone Joint Surg. Br. May 1974;56(2):297-304.